Spectral entropy indicates electrophysiological and hemodynamic changes in drug-resistant epilepsy - A multimodal MREG study
- PMID: 30927607
- PMCID: PMC6444290
- DOI: 10.1016/j.nicl.2019.101763
Spectral entropy indicates electrophysiological and hemodynamic changes in drug-resistant epilepsy - A multimodal MREG study
Abstract
Objective: Epilepsy causes measurable irregularity over a range of brain signal frequencies, as well as autonomic nervous system functions that modulate heart and respiratory rate variability. Imaging dynamic neuronal signals utilizing simultaneously acquired ultra-fast 10 Hz magnetic resonance encephalography (MREG), direct current electroencephalography (DC-EEG), and near-infrared spectroscopy (NIRS) can provide a more comprehensive picture of human brain function. Spectral entropy (SE) is a nonlinear method to summarize signal power irregularity over measured frequencies. SE was used as a joint measure to study whether spectral signal irregularity over a range of brain signal frequencies based on synchronous multimodal brain signals could provide new insights in the neural underpinnings of epileptiform activity.
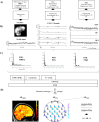
Methods: Ten patients with focal drug-resistant epilepsy (DRE) and ten healthy controls (HC) were scanned with 10 Hz MREG sequence in combination with EEG, NIRS (measuring oxygenated, deoxygenated, and total hemoglobin: HbO, Hb, and HbT, respectively), and cardiorespiratory signals. After pre-processing, voxelwise SEMREG was estimated from MREG data. Different neurophysiological and physiological subfrequency band signals were further estimated from MREG, DC-EEG, and NIRS: fullband (0-5 Hz, FB), near FB (0.08-5 Hz, NFB), brain pulsations in very-low (0.009-0.08 Hz, VLFP), respiratory (0.12-0.4 Hz, RFP), and cardiac (0.7-1.6 Hz, CFP) frequency bands. Global dynamic fluctuations in MREG and NIRS were analyzed in windows of 2 min with 50% overlap.
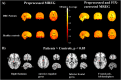
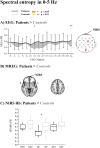
Results: Right thalamus, cingulate gyrus, inferior frontal gyrus, and frontal pole showed significantly higher SEMREG in DRE patients compared to HC. In DRE patients, SE of cortical Hb was significantly reduced in FB (p = .045), NFB (p = .017), and CFP (p = .038), while both HbO and HbT were significantly reduced in RFP (p = .038, p = .045, respectively). Dynamic SE of HbT was reduced in DRE patients in RFP during minutes 2 to 6. Fitting to the frontal MREG and NIRS results, DRE patients showed a significant increase in SEEEG in FB in fronto-central and parieto-occipital regions, in VLFP in parieto-central region, accompanied with a significant decrease in RFP in frontal pole and parietal and occipital (O2, Oz) regions.
Conclusion: This is the first study to show altered spectral entropy from synchronous MREG, EEG, and NIRS in DRE patients. Higher SEMREG in DRE patients in anterior cingulate gyrus together with SEEEG and SENIRS results in 0.12-0.4 Hz can be linked to altered parasympathetic function and respiratory pulsations in the brain. Higher SEMREG in thalamus in DRE patients is connected to disturbances in anatomical and functional connections in epilepsy. Findings suggest that spectral irregularity of both electrophysiological and hemodynamic signals are altered in specific way depending on the physiological frequency range.
Keywords: EEG; Epilepsy; Irregularity; NIRS; Parasympathetic; Ultra-fast fMRI.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Aarabi A., Fazel-Rezai R., Aghakhani Y. A fuzzy rule-based system for epileptic seizure detection in intracranial EEG. Clin. Neurophysiol. 2009;120:1648–1657. - PubMed
-
- Adelson P.D., Nemoto E., Scheuer M., Painter M., Morgan J., Yonas H. Noninvasive continuous monitoring of cerebral oxygenation periictally using near-infrared spectroscopy: a preliminary report. Epilepsia. 1999;40:1484–1489. - PubMed
-
- Allen P.J., Polizzi G., Krakow K., Fish D.R., Lemieux L. Identification of EEG events in the MR scanner: the problem of pulse artifact and a method for its subtraction. Neuroimage. 1998;8:229–239. - PubMed
-
- Allen P.J., Josephs O., Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12:230–239. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

