Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer
- PMID: 30927911
- PMCID: PMC6441211
- DOI: 10.1186/s12943-019-0972-8
Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer
Abstract
Background: Although the tumor stroma in solid tumors like gastric cancer (GC) plays a crucial role in chemo-resistance, specific targets to inhibit the interaction between the stromal and cancer cells have not yet been utilized in clinical practice. The present study aims to determine whether cancer-associated fibroblasts (CAFs), a major component of the tumor stroma, confer chemotherapeutic resistance to GC cells, and to discover potential targets to improve chemo-response in GC.
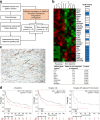
Methods: To identify CAF-specific proteins and signal transduction pathways affecting chemo-resistance in GC cells, secretome and transcriptome analyses were performed. We evaluated the inhibiting effect of CAF-specific protein in in vivo and in vitro models and investigated the expression of CAF-specific protein in human GC tissues.
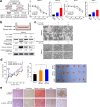
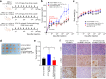
Results: Secretome and transcriptome data revealed that interleukin-6 (IL-6) is a CAF-specific secretory protein that protects GC cells via paracrine signaling. Furthermore, CAF-induced activation of the Janus kinase 1-signal transducer and activator of transcription 3 signal transduction pathway confers chemo-resistance in GC cells. CAF-mediated inhibition of chemotherapy-induced apoptosis was abrogated by the anti-IL-6 receptor monoclonal antibody tocilizumab in various experimental models. Clinical data revealed that IL-6 was prominently expressed in the stromal portion of GC tissues, and IL-6 upregulation in GC tissues was correlated with poor responsiveness to chemotherapy.
Conclusions: Our data provide plausible evidence for crosstalk between GC cells and CAFs, wherein IL-6 is a key contributor to chemoresistance. These findings suggest the potential therapeutic application of IL-6 inhibitors to enhance the responsiveness to chemotherapy in GC.
Keywords: Cancer-associated fibroblasts; Chemo-resistance; Gastric cancer; Interleukin-6; Jak1-STAT3; Tocilizumab; Tumor microenvironment.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the institutional review board/ethics committee of the Ajou University Hospital (AJIRB-BMR-KSP-15-432).
Consent for publication
Not applicable.
Competing interests
R.A. Brekken has an ownership interest (including stock, patents, etc.) in and is a consultant/advisory board member for Tuevol Therapeutics. Other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom H, Heuman R. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/A:1008243606668. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

