Stem cells in homeostasis and cancer of the gut
- PMID: 30927915
- PMCID: PMC6441158
- DOI: 10.1186/s12943-019-0962-x
Stem cells in homeostasis and cancer of the gut
Abstract
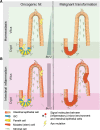
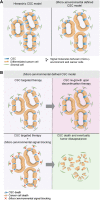
The intestinal epithelial lining is one of the most rapidly renewing cell populations in the body. As a result, the gut has been an attractive model to resolve key mechanisms in epithelial homeostasis. In particular the role of intestinal stem cells (ISCs) in the renewal process has been intensely studied. Interestingly, as opposed to the traditional stem cell theory, the ISC is not a static population but displays significant plasticity and in situations of tissue regeneration more differentiated cells can revert back to a stem cell state upon exposure to extracellular signals. Importantly, normal intestinal homeostasis provides important insight into mechanisms that drive colorectal cancer (CRC) development and growth. Specifically, the dynamics of cancer stem cells bear important resemblance to ISC functionality. In this review we present an overview of the current knowledge on ISCs in homeostasis and their role in malignant transformation. Also, we discuss the existence of stem cells in intestinal adenomas and CRC and how these cells contribute to (pre-)malignant growth. Furthermore, we will focus on new paradigms in the field of dynamical cellular hierarchies in CRC and the intimate relationship between tumor cells and their niche.
Keywords: Cancer stem cells; Cell plasticity; Colorectal cancer; Intestinal stem cells; Tumor (micro-)environment.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, et al. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature. 2017;543(7647):676–680. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

