Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis
- PMID: 30927925
- PMCID: PMC6441214
- DOI: 10.1186/s12943-019-0976-4
Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis
Abstract
Background: Tumor-associated macrophages (TAMs) are major components of tumor microenvironment that frequently associated with tumor metastasis in human cancers. Circulating tumor cell (CTC), originating from primary tumor sites, is considered to be the precursors of tumor metastasis. However, the regulatory mechanism of TAMs in CTC-mediated tumor metastasis still remains unclear.
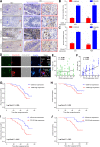
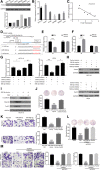
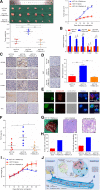
Methods: Immunohistochemical staining was used to detect the macrophages infiltration (CD68 and CD163), epithelial-mesenchymal transition (EMT) markers (E-cadherin and Vimentin) expression in serial sections of human colorectal cancer (CRC) specimens. Then, the correlations between macrophages infiltration and clinicopathologic features, mesenchymal CTC ratio, and patients' prognosis were analyzed. A co-culture assay in vitro was used to evaluate the role of TAMs on CRC EMT, migration and invasion, and ELISA, luciferase reporter assay and CHIP were performed to uncover the underlying mechanism. Furthermore, an in vivo model was carried out to confirm the effect of TAMs on mesenchymal CTC-mediated metastasis.
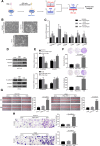
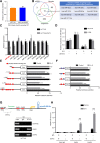
Results: Clinically, CD163+ TAMs infiltrated in invasive front was associated with EMT, mesenchymal CTC ratio, and poor prognosis in patients with CRC. CRC-conditioned macrophages regulated EMT program to enhance CRC cells migration and invasion by secreting IL6. TAMs-derived IL6 activated the JAK2/STAT3 pathway, and activated STAT3 transcriptionally inhibited the tumor suppressor miR-506-3p in CRC cells. miR-506-3p, a key miRNA regulating FoxQ1, was downregulated in CRC cells, resulting in increased FoxQ1 expression, which in turn led to the production of CCL2 that promoted macrophage recruitment. Inhibition of CCL2 or IL6 broke this loop and reduced macrophage migration and mesenchymal CTC-mediated metastasis, respectively.
Conclusions: Our data indicates that TAMs induce EMT program to enhance CRC migration, invasion, and CTC-mediated metastasis by regulating the JAK2/STAT3/miR-506-3p/FoxQ1 axis, which in turn leads to the production of CCL2 that promote macrophage recruitment, revealing a new cross-talk between immune cells and tumor cells in CRC microenvironment.
Keywords: Circulating tumor cell; Colorectal cancer; EMT; Metastasis; Prognosis; Tumor-associated macrophages.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Zhongnan Hospital of Wuhan University ethics committee and written informed consent was obtained from all patients. All animal studies were approved by the Institutional Animal Care and Use Committee of the Zhongnan Hospital of Wuhan university.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

