Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE)
- PMID: 30928825
- PMCID: PMC6535121
- DOI: 10.1016/j.ahj.2018.12.016
Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE)
Abstract
Background: Cardiovascular disease (CVD) is more frequent among people with HIV (PWH) and may relate to traditional and nontraditional factors, including inflammation and immune activation. A critical need exists to develop effective strategies to prevent CVD in this population.
Methods: The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) (A5332) is a prospective, randomized, placebo-controlled trial of a statin strategy for the primary prevention of major adverse cardiovascular events (MACE) in PWH with low to moderate traditional risk. At least 7,500 PWH, 40-75 years of age, on stable antiretroviral therapy, will be randomized to pitavastatin calcium (4 mg/d) or identical placebo and followed for up to 8 years. Participants are enrolled based on the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) atherosclerotic cardiovascular disease (ASCVD) risk score and low-density lipoprotein cholesterol (LDL-C) level with a goal to identify a low- to moderate-risk population who might benefit from a pharmacologic CVD prevention strategy. Potential participants with a risk score ≤ 15% were eligible based on decreasing LDL-C thresholds for increasing risk score >7.5% (LDL-C <190 mg/dL for risk score <7.5%, LDL-C <160 mg/dL for risk score 7.6%-10%, and LDL-C<130 mg/dL for risk score 10.1%-15%). The primary objective is to determine effects on a composite end point of MACE. Formal and independent adjudication of clinical events will occur using standardized criteria. Key secondary end points include effects on MACE components, all-cause mortality, specified non-CVD events, AIDS and non-AIDS events, and safety.
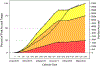
Results: To date, REPRIEVE has enrolled >7,500 participants at approximately 120 sites across 11 countries, generating a diverse and representative population of PWH to investigate the primary objective of the trial.
Conclusions: REPRIEVE is the first trial investigating a primary CVD prevention strategy in PWH. REPRIEVE will inform the field of the efficacy and safety of a statin strategy among HIV-infected participants on antiretroviral therapy and provide critical information on CVD mechanisms and non-CVD events in PWH.
Trial registration: ClinicalTrials.gov NCT02344290.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



References
-
- UNAIDS. Fact Sheet - Latest Statistics on the status of the AIDS epidemic. In: UNAIDS; 2017. pp. Fact Sheet.
-
- UNAIDS/WHO 2010 AIDS Epidemic Update. In UNAIDS Report on the Global AIDS Epidemic; 2010.
-
- Klein D, Hurley LB, Quesenberry CP Jr., Sidney S Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr 2002,30:471–477. - PubMed
-
- Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary Heart Disease in HIV-Infected Individuals. J Acquir Immune Defic Syndr 2003,33:506–512. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069494/AI/NIAID NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- U01 HL123336/HL/NHLBI NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- U01 HL123339/HL/NHLBI NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States

