Methodological advantages and disadvantages of parallel and crossover randomised clinical trials on methylphenidate for attention deficit hyperactivity disorder: a systematic review and meta-analyses
- PMID: 30928951
- PMCID: PMC6475340
- DOI: 10.1136/bmjopen-2018-026478
Methodological advantages and disadvantages of parallel and crossover randomised clinical trials on methylphenidate for attention deficit hyperactivity disorder: a systematic review and meta-analyses
Abstract
Objective: To assess the methodological advantages and disadvantages of parallel and crossover designs in randomised clinical trials on methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD).
Design: Secondary analyses of a Cochrane systematic review.
Setting and participants: We searched relevant databases up to March 2015 and included data from parallel and crossover randomised trials assessing children and adolescents up to 18 years with ADHD.
Interventions: Methylphenidate compared with placebo or no-treatment interventions.
Primary and secondary outcomes: The primary outcomes were teacher-rated ADHD symptoms and serious adverse events. The secondary outcomes were non-serious adverse events.
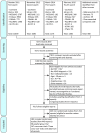
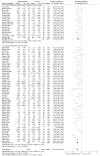
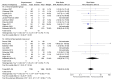
Results: We included 38 parallel trials (n=5111) and 147 crossover trials (n=7134). When comparing methylphenidate with placebo or no-treatment on ADHD symptoms, we found no differences between the end of parallel trials and the first-period from crossover trials (Χ²=1.06, df=1, p=0.30, I²=5.5%). We also found no differences when combining the end of first-period crossover trials with the end of parallel trials and comparing them to the end of last-period crossover trials (Χ²=3.25, df=1, p=0.07, I²=69.2%). We found no differences in serious and non-serious adverse events, and no risk of period and carryover effects. However, only two trials contributed data to the latter analyses.
Conclusions: Both parallel and crossover trials seem suitable for investigating methylphenidate in children and adolescents with ADHD, with comparable estimates on ADHD symptom severity and adverse events. However, parallel trials might still offer ethical and statistical advantages over crossover trials.
Keywords: adhd; attention deficit hyperactivity disorder; crossover trial; methylphenidate; parallel trial.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V). 5th Ed Arlington, Va: American Psychiatric Association, 2013.
-
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines, 2004.
-
- NICE. Attention deficit hyperactivity disorder: diagnosis and management (NG87). National institute for health and care excellence. 2018. nice.org.uk/guidance/ng87 (accessed 13 Nov 2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
