The effect of luseogliflozin and alpha-glucosidase inhibitor on heart failure with preserved ejection fraction in diabetic patients: rationale and design of the MUSCAT-HF randomised controlled trial
- PMID: 30928954
- PMCID: PMC6475163
- DOI: 10.1136/bmjopen-2018-026590
The effect of luseogliflozin and alpha-glucosidase inhibitor on heart failure with preserved ejection fraction in diabetic patients: rationale and design of the MUSCAT-HF randomised controlled trial
Erratum in
-
Correction: The effect of luseogliflozin and alpha-glucosidase inhibitor on heart failure with preserved ejection fraction in diabetic patients: rationale and design of the MUSCAT-HF randomised controlled trial.BMJ Open. 2019 Jun 1;9(5):e026590corr1. doi: 10.1136/bmjopen-2018-026590corr1. BMJ Open. 2019. PMID: 31154319 Free PMC article. No abstract available.
Abstract
Introduction: Type 2 diabetes mellitus (T2DM) is a strong risk factor for coronary artery disease and heart failure, particularly heart failure with preserved ejection fraction (HFpEF). The aim of the ongoing MUSCAT-HF (It stands for Prospective Comparison of Luseogliflozin and Alpha-glucosidase on the Management of Diabetic Patients with Chronic Heart Failure and Preserved Ejection Fraction) trial is to evaluate the efficacy of luseogliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, versus voglibose, an alpha-glucosidase inhibitor, using brain natriuretic peptide (BNP) as the index of therapeutic effect in T2DM patients with HFpEF.
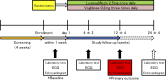
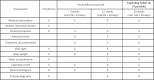
Methods and analysis: A total of 190 patients with T2DM and HFpEF (ejection fraction >45%) who are drug-naïve or taking any anti-diabetic agents will be randomised (1:1) to receive luseogliflozin 2.5 mg one time per day or voglibose 0.2 mg three times per day. The patients will be stratified by age (<65 years, ≥65 years), baseline haemoglobin A1c (<8.0%, ≥8.0%), baseline BNP (<100 pg/mL, ≥100 pg/mL), baseline renal function (estimated glomerular filtration rate ≥60 mL/min/1.73 m2, <60 mL/min/1.73 m2), use of thiazolidine or not and presence or absence of atrial fibrillation and flutter at screening. After randomisation, participants will receive the study drug for 12 weeks in addition to their background therapy. The primary endpoint is the proportional change in baseline BNP after 12 weeks of treatment. The key secondary endpoints are the change from baseline in the ratio of early mitral inflow velocity to mitral annular early diastolic velocity, body weight and glycaemic control after 12 weeks of treatment.
Ethics and dissemination: The study has been approved by the ethics committee and the patients will be included after informed consent. The results will be submitted for publication in peer-reviewed journals.
Trial registration number: UMIN000018395.
Keywords: brain natriuretic peptide; heart failure; luseogliflozin; sodium-glucose cotransporter 2 inhibitor; type 2 diabetes mellitus; voglibose.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KE, SS, MM, SN and AT have no competing interests to declare. TM and KN has received honorarium from Novartis Pharmaceuticals (Basel, Switzerland). HI has received research funding and honorarium from Novartis Pharmaceuticals (Basel, Switzerland).
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
