In Vitro Granuloma Models of Tuberculosis: Potential and Challenges
- PMID: 30929010
- PMCID: PMC6534193
- DOI: 10.1093/infdis/jiz020
In Vitro Granuloma Models of Tuberculosis: Potential and Challenges
Abstract
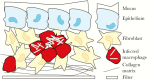
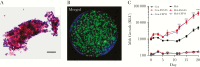
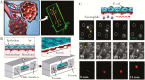
Despite intensive research efforts, several fundamental disease processes for tuberculosis (TB) remain poorly understood. A central enigma is that host immunity is necessary to control disease yet promotes transmission by causing lung immunopathology. Our inability to distinguish these processes makes it challenging to design rational novel interventions. Elucidating basic immune mechanisms likely requires both in vivo and in vitro analyses, since Mycobacterium tuberculosis is a highly specialized human pathogen. The classic immune response is the TB granuloma organized in three dimensions within extracellular matrix. Several groups are developing cell culture granuloma models. In January 2018, NIAID convened a workshop, entitled "3-D Human in vitro TB Granuloma Model" to advance the field. Here, we summarize the arguments for developing advanced TB cell culture models and critically review those currently available. We discuss how integrating complementary approaches, specifically organoids and mathematical modeling, can maximize progress, and conclude by discussing future challenges and opportunities.
Keywords: granuloma; tissue culture models; tuberculosis.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


References
-
- Wallis RS, Maeurer M, Mwaba P, et al. . Tuberculosis–advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect Dis 2016; 16:e34–46. - PubMed
-
- Pagán AJ, Ramakrishnan L. The formation and function of granulomas. Annu Rev Immunol 2018; 36:639–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

