Differentiating Basal Insulin Preparations: Understanding How They Work Explains Why They Are Different
- PMID: 30929185
- PMCID: PMC6824364
- DOI: 10.1007/s12325-019-00925-6
Differentiating Basal Insulin Preparations: Understanding How They Work Explains Why They Are Different
Abstract
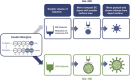
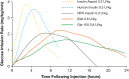
Since the introduction of insulin as a life-saving agent for patients with type 1 diabetes, insulin preparations have evolved to approximate physiologic insulin delivery profiles to meet prandial and basal insulin needs. While prandial insulins are designed to have quick time-action profiles that minimize postprandial glucose excursions, basal insulins are designed to have a protracted time-action profile to facilitate basal glucose control over 24 h. Given that all insulins have the same mechanism of action at the target tissue level, the differences in time-action profiles are achieved through different mechanisms of protraction, resulting in different behaviors in the subcutaneous space and different rates of absorption into the circulation. Herein, we evaluate the differences in basal insulin preparations based on their differential mechanisms of protraction, and the resulting clinical action profiles. Multiple randomized control trials and real-world evidence studies have demonstrated that the newer second-generation basal insulin analogs, insulin glargine 300 units/mL and insulin degludec 100 or 200 units/mL, provide stable glycemic control with once-daily dosing and are associated with a reduced risk of hypoglycemia compared with previous-generation basal insulin analogs insulin glargine 100 units/mL and insulin detemir. These advantages can lead to decreased healthcare resource utilization and cost. With this collective knowledge, healthcare providers and payers can make educated and well-informed decisions when determining which treatment regimen best meets the needs of each individual patient.Funding: Sanofi US, Inc.
Keywords: Basal insulin; Hypoglycemia; Pharmacodynamics; Pharmacokinetics; Protraction; Second-generation long-acting insulin.
Figures





References
-
- Anderson JE. An evolutionary perspective on basal insulin in diabetes treatment: innovations in insulin: insulin glargine U-300. J Fam Pract. 2016;65:S23–S28. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

