Variability in the Analgesic Response to Ibuprofen Is Associated With Cyclooxygenase Activation in Inflammatory Pain
- PMID: 30929268
- PMCID: PMC6753944
- DOI: 10.1002/cpt.1446
Variability in the Analgesic Response to Ibuprofen Is Associated With Cyclooxygenase Activation in Inflammatory Pain
Abstract
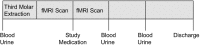
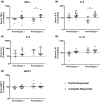
The mechanisms underlying interindividual variability in analgesic efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) are not well understood. Therefore, we performed pain phenotyping, functional neuroimaging, pharmacokinetic/pharmacodynamic assessments, inflammation biomarkers, and gene expression profiling in healthy subjects who underwent surgical extraction of bony impacted third molars and were treated with ibuprofen (400 mg; N = 19) or placebo (N = 10). Analgesic efficacy was not associated with demographic or clinical characteristics, ibuprofen pharmacokinetics, or the degree of cyclooxygenase inhibition by ibuprofen. Compared with partial responders to ibuprofen (N = 9, required rescue medication within the dosing interval), complete responders (N = 10, no rescue medication) exhibited greater induction of urinary prostaglandin metabolites and serum tumor necrosis factor-α and interleukin 8. Differentially expressed genes in peripheral blood mononuclear cells were enriched for inflammation-related pathways. These findings suggest that a less pronounced activation of the inflammatory prostanoid system is associated with insufficient pain relief on ibuprofen alone and the need for additional therapeutic intervention.
© 2019 The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
J.T.F. has received research support from the US Food and Drug Administration, and the National Institutes of Health, Data Safety Monitoring Board service compensation from National Institutes of Health and Cara Therapeutics, and consulting fees from Analgesic Solutions, Aptinyx, Biogen, Campbell Alliance, Daiichi Sankyo, DepoMed, Evadera, Jansen, Lilly, Novartis, and Pfizer. T.G. has received consulting fees from Novartis, Bayer, and PLx Pharma. G.A.F. received research support from the National Institutes of Health, the American Heart Association, the Volkswagen Foundation, Calico Laboratories, and Amgen. He received consulting fees from Amgen, GSK, Tremeau Pharmaceuticals, and Heron Therapeutics. E.V.H. received research funding from Pfizer and Consumer Healthcare Products Association. X.L. became an employee of Eli Lilly and Merck following completion of his work on this study. All other authors declared no competing interests for this work.
Figures



References
-
- Thiels, C.A. et al Wide variation and overprescription of opioids after elective surgery. Ann. Surg. 266, 564–573 (2017). - PubMed
-
- Gauger, E.M. , Gauger, E.J. , Desai, M.J. & Lee, D.H. Opioid use after upper extremity surgery. J. Hand Surg. Am. 43, 470–479 (2018). - PubMed
-
- Chen, L. , Yang, G. & Grosser, T. Prostanoids and inflammatory pain. Prostaglandins Other Lipid. Mediat. 104–105, 58–66 (2013). - PubMed
-
- Grosser, T. , Theken, K.N. & FitzGerald, G.A. Cyclooxygenase inhibition: pain, inflammation, and the cardiovascular system. Clin. Pharmacol. Ther. 102, 611–622 (2017). - PubMed
-
- Bickel, A. , Dorfs, S. , Schmelz, M. , Forster, C. , Uhl, W. & Handwerker, H.O. Effects of antihyperalgesic drugs on experimentally induced hyperalgesia in man. Pain 76, 317–325 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

