Steroid Hormone Function Controls Non-competitive Plasmodium Development in Anopheles
- PMID: 30929905
- PMCID: PMC6450776
- DOI: 10.1016/j.cell.2019.02.036
Steroid Hormone Function Controls Non-competitive Plasmodium Development in Anopheles
Abstract
Transmission of malaria parasites occurs when a female Anopheles mosquito feeds on an infected host to acquire nutrients for egg development. How parasites are affected by oogenetic processes, principally orchestrated by the steroid hormone 20-hydroxyecdysone (20E), remains largely unknown. Here we show that Plasmodium falciparum development is intimately but not competitively linked to processes shaping Anopheles gambiae reproduction. We unveil a 20E-mediated positive correlation between egg and oocyst numbers; impairing oogenesis by multiple 20E manipulations decreases parasite intensities. These manipulations, however, accelerate Plasmodium growth rates, allowing sporozoites to become infectious sooner. Parasites exploit mosquito lipids for faster growth, but they do so without further affecting egg development. These results suggest that P. falciparum has adopted a non-competitive evolutionary strategy of resource exploitation to optimize transmission while minimizing fitness costs to its mosquito vector. Our findings have profound implications for currently proposed control strategies aimed at suppressing mosquito populations.
Keywords: 20E signaling; Anopheles-Plasmodium interactions; EIP; extrinsic incubation period; lipid transport; trade-offs.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests
Figures
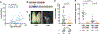



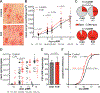

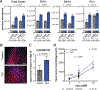
Comment in
-
Coalition Politics: Linking Malaria Transmission to Mosquito Reproduction.Trends Parasitol. 2019 Jul;35(7):486-489. doi: 10.1016/j.pt.2019.05.003. Epub 2019 May 25. Trends Parasitol. 2019. PMID: 31138514
-
Parasitism: Anopheles Mosquitoes and Plasmodium Parasites Share Resources.Curr Biol. 2019 Jul 8;29(13):R632-R634. doi: 10.1016/j.cub.2019.05.030. Curr Biol. 2019. PMID: 31287981
References
-
- Ahmed AM, Maingon RD, Taylor PJ, and Hurd H (1999). The effects of infection with Plasmodium yoelii nigeriensis on the reproductive fitness of the mosquito Anopheles gambiae. Invertebr Repr Dev 36, 217–222.
-
- Arrighi RB, Lycett G, Mahairaki V, Siden-Kiamos I, and Louis C (2005). Laminin and the malaria parasite’s journey through the mosquito midgut. J Exp Biol 208, 2497–2502. - PubMed
-
- Atella GC, Bittencourt-Cunha PR, Nunes RD, Shahabuddin M, and Silva-Neto MA (2009). The major insect lipoprotein is a lipid source to mosquito stages of malaria parasite. Acta Trop 109, 159–162. - PubMed
-
- Attardo GM, Hansen IA, and Raikhel AS (2005). Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem Mol Biol 35, 661–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

