The myristoylated alanine-rich C kinase substrate differentially regulates kinase interacting with stathmin in vascular smooth muscle and endothelial cells and potentiates intimal hyperplasia formation
- PMID: 30929966
- PMCID: PMC6765458
- DOI: 10.1016/j.jvs.2018.12.022
The myristoylated alanine-rich C kinase substrate differentially regulates kinase interacting with stathmin in vascular smooth muscle and endothelial cells and potentiates intimal hyperplasia formation
Abstract
Objective: Restenosis limits the durability of all cardiovascular reconstructions. Vascular smooth muscle cell (VSMC) proliferation drives this process, but an intact, functional endothelium is necessary for vessel patency. Current strategies to prevent restenosis employ antiproliferative agents that affect both VSMCs and endothelial cells (ECs). Knockdown of the myristoylated alanine-rich C kinase substrate (MARCKS) arrests VSMC proliferation and paradoxically potentiates EC proliferation. MARCKS knockdown decreases expression of the kinase interacting with stathmin (KIS), increasing p27kip1 expression, arresting VSMC proliferation. Here, we seek to determine how MARCKS influences KIS protein expression in these two cell types.
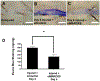
Methods: Primary human coronary artery VSMCs and ECs were used for in vitro experiments. MARCKS was depleted by transfection with small interfering RNA. Messenger RNA was quantitated with the real-time reverse transcription polymerase chain reaction. Protein expression was determined by Western blot analysis. Ubiquitination was determined with immunoprecipitation. MARCKS and KIS binding was assessed with co-immunoprecipitation. Intimal hyperplasia was induced in CL57/B6 mice with a femoral artery wire injury. MARCKS was knocked down in vivo by application of 10 μM of small interfering RNA targeting MARCKS suspended in 30% Pluronic F-127 gel. Intimal hyperplasia formation was assessed by measurement of the intimal thickness on cross sections of the injured artery. Re-endothelialization was determined by quantitating the binding of Evans blue dye to the injured artery.
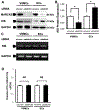
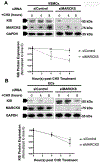
Results: MARCKS knockdown did not affect KIS messenger RNA expression in either cell type. In the presence of cycloheximide, MARCKS knockdown in VSMCs decreased KIS protein stability but had no effect in ECs. The effect of MARCKS knockdown on KIS stability was abrogated by the 26s proteasome inhibitor MG-132. MARCKS binds to KIS in VSMCs but not in ECs. MARCKS knockdown significantly increased the level of ubiquitinated KIS in VSMCs but not in ECs. MARCKS knockdown in vivo resulted in decreased KIS expression. Furthermore, MARCKS knockdown in vivo resulted in decreased 5-ethynyl-2'-deoxyuridine integration and significantly reduced intimal thickening. MARCKS knockdown enhanced endothelial barrier function recovery 4 days after injury.
Conclusions: MARCKS differentially regulates the KIS protein stability in VSMCs and ECs. The difference in stability is due to differential ubiquitination of KIS in these two cell types. The differential interaction of MARCKS and KIS provides a possible explanation for the observed difference in ubiquitination. The effect of MARCKS knockdown on KIS expression persists in vivo, potentiates recovery of the endothelium, and abrogates intimal hyperplasia formation.
Keywords: Cell migration; Cell proliferation; KIS; MARCKS.
Copyright © 2019 Society for Vascular Surgery. Published by Elsevier Inc. All rights reserved.
Figures



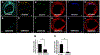
Comment in
-
Invited commentary.J Vasc Surg. 2019 Dec;70(6):2031-2032. doi: 10.1016/j.jvs.2018.12.021. J Vasc Surg. 2019. PMID: 31761108 No abstract available.
References
-
- Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Tielbeek A, et al. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J Vasc Interv Radiol. 2005;16(3):331–8. - PubMed
-
- Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwalder U, Beregi JP, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358(7):689–99. - PubMed
-
- Holmes DR Jr., Leon MB, Moses JW, Popma JJ, Cutlip D, Fitzgerald PJ, et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. 2004;109(5):634–40. - PubMed
-
- Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007; 115(18):2435–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

