Interactions Between Commensal Bacteria and Enteric Neurons, via FPR1 Induction of ROS, Increase Gastrointestinal Motility in Mice
- PMID: 30930024
- PMCID: PMC8733963
- DOI: 10.1053/j.gastro.2019.03.045
Interactions Between Commensal Bacteria and Enteric Neurons, via FPR1 Induction of ROS, Increase Gastrointestinal Motility in Mice
Abstract
Background & aims: Reduced gastrointestinal (GI) motility is a feature of disorders associated with intestinal dysbiosis and loss of beneficial microbes. It is not clear how consumption of beneficial commensal microbes, marketed as probiotics, affects the enteric nervous system (ENS). We studied the effects of the widely used probiotic and the commensal Lactobacillus rhamnosus GG (LGG) on ENS and GI motility in mice.
Methods: Conventional and germ free C57B6 mice were gavaged with LGG and intestinal tissues were collected; changes in the enteric neuronal subtypes were assessed by real-time polymerase chain reaction, immunoblots, and immunostaining. Production of reactive oxygen species (ROS) in the jejunal myenteric plexi and phosphorylation (p) of mitogen-activated protein kinase 1 (MAPK1) in the enteric ganglia were assessed by immunoblots and immunostaining. Fluorescence in situ hybridization was performed on jejunal cryosections with probes to detect formyl peptide receptor 1 (FPR1). GI motility in conventional mice was assessed after daily gavage of LGG for 1 week.
Results: Feeding of LGG to mice stimulated myenteric production of ROS, increased levels of phosphorylated MAPK1, and increased expression of choline acetyl transferase by neurons (P < .001). These effects were not observed in mice given N-acetyl cysteine (a ROS inhibitor) or LGGΩSpaC (an adhesion-mutant strain of LGG) or FPR1-knockout mice. Gavage of mice with LGG for 1 week significantly increased stool frequency, reduced total GI transit time, and increased contractions of ileal circular muscle strips in ex vivo experiments (P < .05).
Conclusions: Using mouse models, we found that LGG-mediated signaling in the ENS requires bacterial adhesion, redox mechanisms, and FPR1. This pathway might be activated to increase GI motility in patients.
Keywords: Digestion; ERK; Microbiome; Signal Transduction.
Copyright © 2019 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest- None
Figures




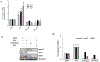
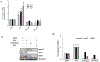





References
-
- Furness JB, Callaghan BP, Rivera LR, et al.. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 2014;817:39–71. - PubMed
-
- McVey Neufeld KA, Mao YK, Bienenstock J, et al.. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil 2013;25:183–e88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

