Evaluation of the cost and effectiveness of diverse recruitment methods for a genetic screening study
- PMID: 30930462
- PMCID: PMC7213972
- DOI: 10.1038/s41436-019-0497-y
Evaluation of the cost and effectiveness of diverse recruitment methods for a genetic screening study
Erratum in
-
Correction: Evaluation of the cost and effectiveness of diverse recruitment methods for a genetic screening study.Genet Med. 2019 Oct;21(10):2407. doi: 10.1038/s41436-019-0528-8. Genet Med. 2019. PMID: 31040387 Free PMC article.
Abstract
Purpose: Recruitment of participants from diverse backgrounds is crucial to the generalizability of genetic research, but has proven challenging. We retrospectively evaluated recruitment methods used for a study on return of genetic results.
Methods: The costs of study design, development, and participant enrollment were calculated, and the characteristics of the participants enrolled through the seven recruitment methods were examined.
Results: A total of 1118 participants provided consent, a blood sample, and questionnaire data. The estimated cost across recruitment methods ranged from $579 to $1666 per participant and required a large recruitment team. Recruitment methods using flyers and staff networks were the most cost-efficient and resulted in the highest completion rate. Targeted sampling that emphasized the importance of Latino/a participation, utilization of translated materials, and in-person recruitments contributed to enrolling a demographically diverse sample.
Conclusions: Although all methods were deployed in the same hospital or neighborhood and shared the same staff, each recruitment method was different in terms of cost and characteristics of the enrolled participants, suggesting the importance of carefully choosing the recruitment methods based on the desired composition of the final study sample. This analysis provides information about the effectiveness and cost of different methods to recruit adults for genetic research.
Keywords: cost-efficiency; genomic research; personalized medicine; recruitment methods; socioeconomic diversity.
Conflict of interest statement
Conflict of Interests:
The authors have no conflict of interests to report.
Figures

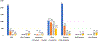
References
-
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Sciences Policy, Roundtable on Genomics and Precision Health. Implementing and Evaluating Genomic Screening Programs in Health Care Systems: Proceedings of a Workshop. Washington (DC): National Academies Press (US); 2018. http://www.ncbi.nlm.nih.gov/books/NBK500393/. Accessed December 24, 2018. - PubMed
-
- Hoyo C, Reid ML, Godley PA, Parrish T, Smith L, Gammon M. Barriers and strategies for sustained participation of African-American men in cohort studies. Ethn Dis. 2003;13(4):470–476. - PubMed
-
- Huynh L, Johns B, Liu S-H, Vedula SS, Li T, Puhan MA. Cost-effectiveness of health research study participant recruitment strategies: a systematic review. Clin Trials. 2014;11(5):576–583. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

