Biased Signaling and Allosteric Modulation at the FSHR
- PMID: 30930853
- PMCID: PMC6425863
- DOI: 10.3389/fendo.2019.00148
Biased Signaling and Allosteric Modulation at the FSHR
Abstract
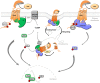
Knowledge on G protein-coupled receptor (GPCRs) structure and mechanism of activation has profoundly evolved over the past years. The way drugs targeting this family of receptors are discovered and used has also changed. Ligands appear to bind a growing number of GPCRs in a competitive or allosteric manner to elicit balanced signaling or biased signaling (i.e., differential efficacy in activating or inhibiting selective signaling pathway(s) compared to the reference ligand). These novel concepts and developments transform our understanding of the follicle-stimulating hormone (FSH) receptor (FSHR) biology and the way it could be pharmacologically modulated in the future. The FSHR is expressed in somatic cells of the gonads and plays a major role in reproduction. When compared to classical GPCRs, the FSHR exhibits intrinsic peculiarities, such as a very large NH2-terminal extracellular domain that binds a naturally heterogeneous, large heterodimeric glycoprotein, namely FSH. Once activated, the FSHR couples to Gαs and, in some instances, to other Gα subunits. G protein-coupled receptor kinases and β-arrestins are also recruited to this receptor and account for its desensitization, trafficking, and intracellular signaling. Different classes of pharmacological tools capable of biasing FSHR signaling have been reported and open promising prospects both in basic research and for therapeutic applications. Here we provide an updated review of the most salient peculiarities of FSHR signaling and its selective modulation.
Keywords: G protein; GPCR; bias; follicle-stimulating hormone; reproduction; signaling; trafficking; β-arrestin.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

