Characterization of Subtilin L-Q11, a Novel Class I Bacteriocin Synthesized by Bacillus subtilis L-Q11 Isolated From Orchard Soil
- PMID: 30930878
- PMCID: PMC6429107
- DOI: 10.3389/fmicb.2019.00484
Characterization of Subtilin L-Q11, a Novel Class I Bacteriocin Synthesized by Bacillus subtilis L-Q11 Isolated From Orchard Soil
Abstract
Bacteriocins are peptides or proteins synthesized by bacterial ribosomes that show killing or inhibitory activities against different groups of bacteria. Bacteriocins are considered potential alternatives to traditional antibiotics, preservatives in pharmaceutical and food industries. A strain L-Q11 isolated from orchard soil was phylogenetically characterized as Bacillus subtilis based on 16S rRNA gene sequencing analysis. A novel class I bacteriocin (Subtilin L-Q11), was identified and purified from L-Q11 cell-free supernatant in a four-step procedure, including salt precipitation, cation exchange, gel filtration, and reverse-phase high-performance liquid chromatography (RP-HPLC). The molecular mass (3,552.9 Da) of this novel bacteriocin was determined by Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). The purified Subtilin L-Q11 exhibited optimal features in pH tolerance, thermostability, and sensitivity to proteases. Further, Subtilin L-Q11 showed inhibitory activities against a number of bacteria including some human pathogens and food spoilage bacteria, in particular Staphylococcus aureus. All these important features make this novel bacteriocin a potential candidate for the development of a new antibacterial drug or food preservative in the future.
Keywords: Bacillus subtilis; Subtilin L-Q11; antibacterial activity; antibacterial mechanism; bacteriocin.
Figures

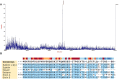
 ” represents the growth curve of L-Q11; “
” represents the growth curve of L-Q11; “ ” represents values of pH in the culture medium; “
” represents values of pH in the culture medium; “ ” represents inhibitory activity against the indicator strain S. aureus ATCC 29213.
” represents inhibitory activity against the indicator strain S. aureus ATCC 29213.

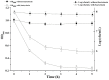
 ” represents viable cell count of untreated S. aureus ATCC 29213; “Δ” represents viable cell count of S. aureus ATCC 29213 with the treatment of bacteriocin; “
” represents viable cell count of untreated S. aureus ATCC 29213; “Δ” represents viable cell count of S. aureus ATCC 29213 with the treatment of bacteriocin; “ ” represents cell optical density at the wavelength of 600 nm (O.D.600) without bacteriocin treatment; “
” represents cell optical density at the wavelength of 600 nm (O.D.600) without bacteriocin treatment; “ ” represents optical density at the wavelength of 600 nm with bacteriocin treatment.
” represents optical density at the wavelength of 600 nm with bacteriocin treatment.
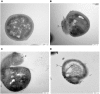
References
-
- An Y., Wang Y., Liang X. Y., Yi H. X., Zuo Z. H., Xu X. X., et al. (2017). Purification and partial characterization of M1-UVs300, a novel bacteriocin produced by Lactobacillus plantarum isolated from fermented sausage. Food Control 81 211–217. 10.1016/j.foodcont.2017.05.030 - DOI
-
- Bressollier P., Brugo M. A., Robineau P., Schmitter J. M., Sofeir M., Urdaci M. C., et al. (2014). “Peptide compound with biological activity, its preparation and its applications”. Google Patents.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

