AMPK-Targeted Effector Networks in Mycobacterial Infection
- PMID: 30930886
- PMCID: PMC6429987
- DOI: 10.3389/fmicb.2019.00520
AMPK-Targeted Effector Networks in Mycobacterial Infection
Abstract
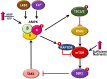
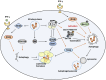
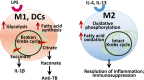
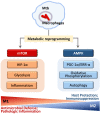
AMP-activated protein kinase (AMPK), a key metabolic regulator, plays an essential role in the maintenance of energy balance in response to stress. Tuberculosis (TB), primarily caused by the pathogen Mycobacterium tuberculosis (Mtb), remains one of the most important infectious diseases worldwide, characterized by both high incidence and mortality. Development of new preventive and therapeutic strategies against TB requires a profound understanding of the various host-pathogen interactions that occur during infection. Emerging data suggest that AMPK plays an essential regulatory role in host autophagy, mitochondrial biogenesis, metabolic reprogramming, fatty acid β-oxidation, and the control of pathologic inflammation in macrophages during Mtb infection. As described in this review, recent studies have begun to define the functional properties of AMPK modulators capable of restricting intracellular bacteria and promoting host defenses. Several host defense factors in the context of AMPK activation also participate in autophagic and non-autophagic pathways in a coordinated manner to enhance antimicrobial responses against Mtb infection. A better understanding of these AMPK-targeted effector networks offers significant potential for the development of novel therapeutics for human TB and other infectious diseases.
Keywords: AMPK; autophagy; immunometabolism; macrophage; mitochondria; mycobacteria.
Figures
References
-
- Baay-Guzman G. J., Duran-Padilla M. A., Rangel-Santiago J., Tirado-Rodriguez B., Antonio-Andres G., Barrios-Payan J., et al. (2018). Dual role of hypoxia-inducible factor 1 alpha in experimental pulmonary tuberculosis: its implication as a new therapeutic target. Future Microbiol. 13 785–798. 10.2217/fmb-2017-0168 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

