Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1
- PMID: 30931312
- PMCID: PMC6423427
- DOI: 10.3389/fmolb.2019.00011
Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1
Abstract
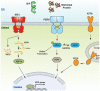
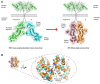
The endoplasmic reticulum (ER) is an important site for protein folding and maturation in eukaryotes. The cellular requirement to synthesize proteins within the ER is matched by its folding capacity. However, the physiological demands or aberrations in folding may result in an imbalance which can lead to the accumulation of misfolded protein, also known as "ER stress." The unfolded protein response (UPR) is a cell-signaling system that readjusts ER folding capacity to restore protein homeostasis. The key UPR signal activator, IRE1, responds to stress by propagating the UPR signal from the ER to the cytosol. Here, we discuss the structural and molecular basis of IRE1 stress signaling, with particular focus on novel mechanistic advances. We draw a comparison between the recently proposed allosteric model for UPR induction and the role of Hsp70 during polypeptide import to the mitochondrial matrix.
Keywords: BiP; ER stress; Hsp70; IRE1 inositol-requiring enzyme 1; crystal structures; unfolded protein response (UPR).
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

