Extending the scope of dispersive liquid-liquid microextraction for trace analysis of 3-methyl-1,2,3-butanetricarboxylic acid in atmospheric aerosols leading to the discovery of iron(III) complexes
- PMID: 30931501
- PMCID: PMC6522453
- DOI: 10.1007/s00216-019-01741-1
Extending the scope of dispersive liquid-liquid microextraction for trace analysis of 3-methyl-1,2,3-butanetricarboxylic acid in atmospheric aerosols leading to the discovery of iron(III) complexes
Abstract
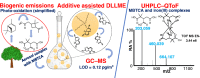
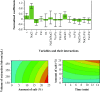
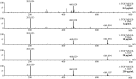
3-Methyl-1,2,3-butanetricarboxylic acid (MBTCA) is a secondary organic aerosol and can be used as a unique emission marker of biogenic emissions of monoterpenes. Seasonal variations and differences in vegetation cover around the world may lead to low atmospheric MBTCA concentrations, in many cases too low to be measured. Hence, an important tool to quantify the contribution of terrestrial vegetation to the loading of secondary organic aerosol may be compromised. To meet this challenge, a dispersive liquid-liquid microextraction (DLLME) method, known for the extraction of hydrophobic compounds, was extended to the extraction of polar organic compounds like MBTCA without compromising the efficiency of the method. The extraction solvent was fine-tuned using tri-n-octyl phosphine oxide as additive. A multivariate experimental design was applied for deeper understanding of significant variables and interactions between them. The optimum extraction conditions included 1-octanol with 15% tri-n-octyl phosphine oxide (w/w) as extraction solvent, methanol as dispersive solvent, 25% NaCl dissolved in 5 mL sample (w/w) acidified to pH 2 using HNO3, and extraction time of 15 min. A limit of detection of 0.12 pg/m3 in air was achieved. Furthermore, unique complexation behavior of MBTCA with iron(III) was found when analyzed with ultra-high-performance liquid chromatography coupled with electrospray ionization-quadrupole time-of-flight mass spectrometry (UHPLC-ESI-QToF). A comprehensive overview of this complexation behavior of MBTCA was examined with systematically designed experiments. This newly discovered behavior of MBTCA will be of interest for further research on organometallic photooxidation chemistry of atmospheric aerosols. Graphical abstract a) Additive assisted DLLME and MBTCA complexes with Fe(III), b) A good quality figure is attached in ppt format to facilitate editable objects.
Keywords: Biogenic secondary organic aerosol; Dispersive liquid–liquid microextraction; MBTCA; Metal complexes; Trace analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, et al. A global model of natural volatile organic compound emissions. J Geophys Res. 1995;100(D5):8873–8892. doi: 10.1029/94JD02950. - DOI
-
- Kourtchev I, Hellebust S, Bell JM, O'Connor IP, Healy RM, et al. The use of polar organic compounds to estimate the contribution of domestic solid fuel combustion and biogenic sources to ambient levels of organic carbon and PM2.5 in Cork Harbour, Ireland. Sci Total Environ. 2011;409(11):2143–2155. doi: 10.1016/j.scitotenv.2011.02.027. - DOI - PubMed
-
- Muller L, Reinnig MC, Naumann KH, Saathoff H, Mentel TF, et al. formation of 3-methyl-1,2,3-butanetricarboxylic acid via gas phase oxidation of pinonic acid - a mass spectrometric study of SOA aging. Atmos Chem Phys. 2012;12(3):1483–96. 10.5194/acp-12-1483-2012.
-
- Kanakidou M, Seinfeld JH, Pandis SN, Barnes I, Dentener FJ, Facchini MC, et al. Organic aerosol and global climate modelling: a review. Atmos Chem Phys. 2005;5:1053–1123. doi: 10.5194/acp-5-1053-2005. - DOI
-
- Szmigielski R, Surratt JD, Gomez-Gonzalez Y, Van der Veken P, Kourtchev I, et al. 3-Methyl-1,2,3-butanetricarboxylic acid: an atmospheric tracer for terpene secondary organic aerosol. Geophys Res Lett. 2007;34(24).
Grants and funding
LinkOut - more resources
Full Text Sources

