Outcomes After Drug-Coated Balloon Treatment of Femoropopliteal Lesions in Patients With Critical Limb Ischemia: A Post Hoc Analysis From the IN.PACT Global Study
- PMID: 30931726
- PMCID: PMC6628633
- DOI: 10.1177/1526602819839044
Outcomes After Drug-Coated Balloon Treatment of Femoropopliteal Lesions in Patients With Critical Limb Ischemia: A Post Hoc Analysis From the IN.PACT Global Study
Abstract
Purpose: To report a post hoc analysis performed to evaluate 1-year safety and efficacy of the IN.PACT Admiral drug-coated balloon (DCB) for the treatment of femoropopliteal lesions in subjects with critical limb ischemia (CLI) enrolled in the IN.PACT Global study ( ClinicalTrials.gov identifier NCT01609296).
Materials and methods: Of 1535 subjects enrolled in the study, 156 participants (mean age 71.8±10.4; 87 men) with CLI (Rutherford categories 4,5) were treated with DCB angioplasty in 194 femoropopliteal lesions. This cohort was compared to the 1246 subjects (mean age 68.2±10.0 years; 864 men) with intermittent claudication (IC) treated for 1573 lesions. The CLI cohort had longer lesions (13.9±10.6 vs 11.9±9.4 cm, p=0.009) and a higher calcification rate (76.8% vs 67.7%, p=0.011). Major adverse events [MAE; composite of all-cause mortality, clinically-driven target lesion revascularization (CD-TLR), major (above-ankle) target limb amputation, and thrombosis at the target lesion site], lesion and vessel revascularization rates, and EuroQol-5D were assessed through 1 year. The Kaplan-Meier method was used to estimate survival, CD-TLR, and amputation events; estimates are presented with the 95% confidence intervals (CI).
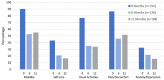
Results: Estimates of 12-month freedom from major target limb amputation were 98.6% (95% CI 96.7% to 100.0%) in subjects with CLI and 99.9% (95% CI 99.8% to 100.0%) in subjects with IC (p=0.002). Freedom from CD-TLR through 12 months was 86.3% (95% CI 80.6% to 91.9%) in CLI subjects and 93.4% (95% CI 91.9% to 94.8%) in IC subjects (p<0.001). The MAE rate through 12 months was higher in CLI subjects (22.5% vs 10.7%, p<0.001), and CLI patients had poorer overall survival (93.0%, 95% CI 88.9% to 97.2%) than IC subjects (97.0%, 95% CI 96.0% to 97.9%, p=0.011). Health status significantly improved in all domains at 6 and 12 months in both groups.
Conclusion: Treatment of femoropopliteal disease with DCB in CLI patients is safe through 12-month follow-up, with a low major amputation rate of 1.4%. The rates of MAE and CD-TLR were higher in CLI subjects and reinterventions were required sooner. Additional research is needed to evaluate long-term outcomes of DCB treatment for femoropopliteal lesions in CLI patients.
Keywords: amputation; claudication; critical limb ischemia; drug-coated balloon; femoropopliteal segment; limb salvage; peripheral artery disease; popliteal artery; stenosis; superficial femoral artery; target lesion revascularization.
Conflict of interest statement
Figures





References
-
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–S67. - PubMed
-
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. - PMC - PubMed
-
- European Stroke Organisation, Tendera M, Aboyans V, et al. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851–2906. - PubMed
-
- Bosma J, Vahl A, Wisselink W. Systematic review on health-related quality of life after revascularization and primary amputation in patients with critical limb ischemia. Ann Vasc Surg. 2013;27:1105–1114. - PubMed
-
- Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol. 2016;19:91–95. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

