iPSCs: A powerful tool for skeletal muscle tissue engineering
- PMID: 30933431
- PMCID: PMC6533516
- DOI: 10.1111/jcmm.14292
iPSCs: A powerful tool for skeletal muscle tissue engineering
Abstract
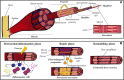
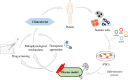
Both volumetric muscle loss (VML) and muscle degenerative diseases lead to an important decrease in skeletal muscle mass, condition that nowadays lacks an optimal treatment. This issue has driven towards an increasing interest in new strategies in tissue engineering, an emerging field that can offer very promising approaches. In addition, the discovery of induced pluripotent stem cells (iPSCs) has completely revolutionized the actual view of personalized medicine, and their utilization in skeletal muscle tissue engineering could, undoubtedly, add myriad benefits. In this review, we want to provide a general vision of the basic aspects to consider when engineering skeletal muscle tissue using iPSCs. Specifically, we will focus on the three main pillars of tissue engineering: the scaffold designing, the selection of the ideal cell source and the addition of factors that can enhance the resemblance with the native tissue.
Keywords: biomaterials; iPS-skeletal muscle; iPSCs; induced pluripotent stem cells; personalized medicine; regenerative medicine; scaffold; tissue engineering; volumetric muscle loss.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors confirm that there is no conflict of interest.
Figures

References
-
- Tabebordbar M, Wang ET, Wagers AJ. Skeletal muscle degenerative diseases and strategies for therapeutic muscle repair. Annu Rev Pathol Mech Dis. 2013;8:441‐475. - PubMed
-
- Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84:822‐832. - PubMed
-
- Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347:759‐774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

