Introduction to Quantitative Susceptibility Mapping and Susceptibility Weighted Imaging
- PMID: 30933548
- PMCID: PMC6732929
- DOI: 10.1259/bjr.20181016
Introduction to Quantitative Susceptibility Mapping and Susceptibility Weighted Imaging
Abstract
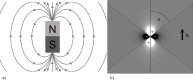
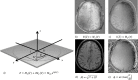
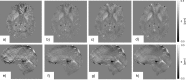
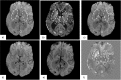
Quantitative Susceptibility Mapping (QSM) and Susceptibility Weighted Imaging (SWI) are MRI techniques that measure and display differences in the magnetization that is induced in tissues, i.e. their magnetic susceptibility, when placed in the strong external magnetic field of an MRI system. SWI produces images in which the contrast is heavily weighted by the intrinsic tissue magnetic susceptibility. It has been applied in a wide range of clinical applications. QSM is a further advancement of this technique that requires sophisticated post-processing in order to provide quantitative maps of tissue susceptibility. This review explains the steps involved in both SWI and QSM as well as describing some of their uses in both clinical and research applications.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

