Natural disease history of the D2 -mdx mouse model for Duchenne muscular dystrophy
- PMID: 30933664
- PMCID: PMC6593893
- DOI: 10.1096/fj.201802488R
Natural disease history of the D2 -mdx mouse model for Duchenne muscular dystrophy
Abstract
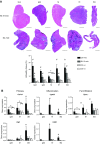
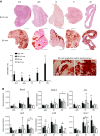
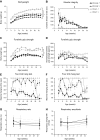
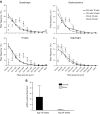
The C57BL/10ScSn-Dmdmdx/J (BL10-mdx) mouse has been the most commonly used model for Duchenne muscular dystrophy (DMD) for decades. Their muscle dysfunction and pathology is, however, less severe than in patients with DMD, which complicates preclinical studies. Recent discoveries indicate that disease severity is exacerbated when muscular dystrophy mouse models are generated on a DBA2/J genetic background. Knowledge on the natural history of animal models is pivotal for high-quality preclinical testing. However, for BL10-mdx mice on a DBA2/J background (D2-mdx), limited data are available. We addressed this gap in the natural history knowledge. First, we compared histopathological aspects in skeletal muscles of young D2-mdx, BL10-mdx, and wild-type mice. Pathology was more pronounced in D2-mdx mice and differed in severity between muscles within individuals. Secondly, we subjected D2-mdx mice to a functional test regime for 34 weeks and identified that female D2-mdx mice outperform severely impaired males, making females less useful for functional preclinical studies. Direct comparisons between 10- and 34-wk-old D2-mdx mice revealed that disease pathology ameliorates with age. Heart pathology was progressive, with some features already evident at a young age. This natural history study of the D2-mdx mouse will be instrumental for experimental design of future preclinical studies.-Van Putten, M., Putker, K., Overzier, M., Adamzek, W. A., Pasteuning-Vuhman, S., Plomp, J. J., Aartsma-Rus, A. Natural disease history of the D2-mdx mouse model for Duchenne muscular dystrophy.
Keywords: calcification; fibrosis; muscle function; pathology; regeneration.
Conflict of interest statement
The authors are grateful for the experimental help of Davy van de Vijver, Mandy Kater, and Joris van der Hurk [Leiden University Medical Centre (LUMC)]. This work was supported by funding from Duchenne Parent Project, French Muscular Dystrophy Association (AFM) Telethon (Grant 20251) and ZonMw (Grant 113302001) for researchers’ salaries. A.A.-R. is employed by Leiden University Medical Center (LUMC), which has patents on exon skipping technology, some of which has been licensed to BioMarin and subsequently sublicensed to Sarepta. As co-inventor of some of these patents, A.A.-R. is entitled to a share of royalties. A.A.-R. is also an
Figures


References
-
- Mendell J. R., Rodino-Klapac L. R., Sahenk Z., Roush K., Bird L., Lowes L. P., Alfano L., Gomez A. M., Lewis S., Kota J., Malik V., Shontz K., Walker C. M., Flanigan K. M., Corridore M., Kean J. R., Allen H. D., Shilling C., Melia K. R., Sazani P., Saoud J. B., Kaye E. M.; Eteplirsen Study Group (2013) Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 74, 637–647 - PubMed
-
- Straub V., Balabanov P., Bushby K., Ensini M., Goemans N., De Luca A., Pereda A., Hemmings R., Campion G., Kaye E., Arechavala-Gomeza V., Goyenvalle A., Niks E., Veldhuizen O., Furlong P., Stoyanova-Beninska V., Wood M. J., Johnson A., Mercuri E., Muntoni F., Sepodes B., Haas M., Vroom E., Aartsma-Rus A. (2016) Stakeholder cooperation to overcome challenges in orphan medicine development: the example of Duchenne muscular dystrophy. Lancet Neurol. 15, 882–890 - PubMed
-
- Emery A. E. (2002) The muscular dystrophies. Lancet 359, 687–695 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

