Targeting the CaVα-CaVβ interaction yields an antagonist of the N-type CaV2.2 channel with broad antinociceptive efficacy
- PMID: 30933958
- PMCID: PMC8998802
- DOI: 10.1097/j.pain.0000000000001524
Targeting the CaVα-CaVβ interaction yields an antagonist of the N-type CaV2.2 channel with broad antinociceptive efficacy
Abstract
Inhibition of voltage-gated calcium (CaV) channels is a potential therapy for many neurological diseases including chronic pain. Neuronal CaV1/CaV2 channels are composed of α, β, γ and α2δ subunits. The β subunits of CaV channels are cytoplasmic proteins that increase the surface expression of the pore-forming α subunit of CaV. We targeted the high-affinity protein-protein interface of CaVβ's pocket within the CaVα subunit. Structure-based virtual screening of 50,000 small molecule library docked to the β subunit led to the identification of 2-(3,5-dimethylisoxazol-4-yl)-N-((4-((3-phenylpropyl)amino)quinazolin-2-yl)methyl)acetamide (IPPQ). This small molecule bound to CaVβ and inhibited its coupling with N-type voltage-gated calcium (CaV2.2) channels, leading to a reduction in CaV2.2 currents in rat dorsal root ganglion sensory neurons, decreased presynaptic localization of CaV2.2 in vivo, decreased frequency of spontaneous excitatory postsynaptic potentials and miniature excitatory postsynaptic potentials, and inhibited release of the nociceptive neurotransmitter calcitonin gene-related peptide from spinal cord. IPPQ did not target opioid receptors nor did it engage inhibitory G protein-coupled receptor signaling. IPPQ was antinociceptive in naive animals and reversed allodynia and hyperalgesia in models of acute (postsurgical) and neuropathic (spinal nerve ligation, chemotherapy- and gp120-induced peripheral neuropathy, and genome-edited neuropathy) pain. IPPQ did not cause akinesia or motor impairment, a common adverse effect of CaV2.2 targeting drugs, when injected into the brain. IPPQ, a quinazoline analog, represents a novel class of CaV2.2-targeting compounds that may serve as probes to interrogate CaVα-CaVβ function and ultimately be developed as a nonopioid therapeutic for chronic pain.
Conflict of interest statement
Conflict of interest statement
R. Khanna, M. Khanna, and V. Gokhale have filed a provisional patent on the use of quinazoline analogs. The remaining authors have no conflicts of interest to declare.
Figures




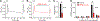
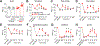

References
-
- Aydin ON, Ek RO, Temoçin S, Uğur B, Alaçam B, Şen S. The antinociceptive effects of systemic administration of tramadol, gabapentin and their combination on mice model of acute pain. Agri 2012;24:49–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

