Platelet-derived growth factor activates nociceptive neurons by inhibiting M-current and contributes to inflammatory pain
- PMID: 30933959
- PMCID: PMC6553959
- DOI: 10.1097/j.pain.0000000000001523
Platelet-derived growth factor activates nociceptive neurons by inhibiting M-current and contributes to inflammatory pain
Abstract
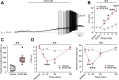
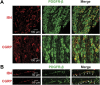
Endogenous inflammatory mediators contribute to the pathogenesis of pain by acting on nociceptors, specialized sensory neurons that detect noxious stimuli. Here, we describe a new factor mediating inflammatory pain. We show that platelet-derived growth factor (PDGF)-BB applied in vitro causes repetitive firing of dissociated nociceptor-like rat dorsal root ganglion neurons and decreased their threshold for action potential generation. Injection of PDGF-BB into the paw produced nocifensive behavior in rats and led to thermal and mechanical pain hypersensitivity. We further detailed the biophysical mechanisms of these PDGF-BB effects and show that PDGF receptor-induced inhibition of nociceptive M-current underlies PDGF-BB-mediated nociceptive hyperexcitability. Moreover, in vivo sequestration of PDGF or inhibition of the PDGF receptor attenuates acute formalin-induced inflammatory pain. Our discovery of a new pain-facilitating proinflammatory mediator, which by inhibiting M-current activates nociceptive neurons and thus contributes to inflammatory pain, improves our understanding of inflammatory pain pathophysiology and may have important clinical implications for pain treatment.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures






References
-
- Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. PAIN 1997;69:101–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

