Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model
- PMID: 30934593
- PMCID: PMC6470567
- DOI: 10.3390/nu11030700
Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model
Abstract
Background: Inflammation and mitochondrial dysfunction have been linked to trauma, neurodegeneration, and aging. Impairment of CISD2 expression may trigger the aforementioned pathological conditions in neural cells. We previously reported that curcumin attenuates the downregulation of CISD2 in animal models of spinal cord injury and lipopolysaccharide (LPS)-treated neuronal cells. In this study, we investigate (1) the role of CISD2 and (2) how curcumin regulates CISD2 in the aging process.
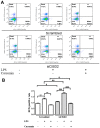
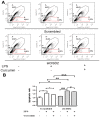
Materials and methods: The serial expression of CISD2 and the efficacy of curcumin treatment were evaluated in old (104 weeks) mice and long-term cultures of neural cells (35 days in vitro, DIV). LPS-challenged neural cells (with or without siCISD2 transfection) were used to verify the role of curcumin on CISD2 underlying mitochondrial dysfunction.
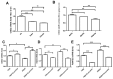
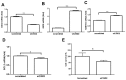
Results: In the brain and spinal cord of mice aged P2, 8, 25, and 104 weeks, we observed a significant decrease in CISD2 expression with age. Curcumin treatment in vivo and in vitro was shown to upregulate CISD2 expression; attenuate inflammatory response in neural cells. Moreover, curcumin treatment elevated CISD2 expression levels and prevented mitochondrial dysfunction in LPS-challenged neural cells. The beneficial effects of curcumin in either non-stressed or LPS-challenged cells that underwent siCISD2 transfection were significantly lower than in respective groups of cells that underwent scrambled siRNA-transfection.
Conclusions: We hypothesize that the protective effects of curcumin treatment in reducing cellular inflammation associated trauma, degenerative, and aging processes can be partially attributed to elevated CISD2 expression. We observed a reduction in the protective effects of curcumin against injury-induced inflammation and mitochondrial dysfunction in cells where CISD2 expression was reduced by siCISD2.
Keywords: CISD2; CISD2-dependent manner; aging; curcumin; neurodegeneration; trauma.
Conflict of interest statement
No competing financial interests exist. The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

