Horse Oil Mitigates Oxidative Damage to Human HaCaT Keratinocytes Caused by Ultraviolet B Irradiation
- PMID: 30934595
- PMCID: PMC6471125
- DOI: 10.3390/ijms20061490
Horse Oil Mitigates Oxidative Damage to Human HaCaT Keratinocytes Caused by Ultraviolet B Irradiation
Abstract
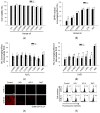
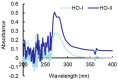
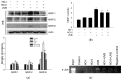
Horse oil products have been used in skin care for a long time in traditional medicine, but the biological effects of horse oil on the skin remain unclear. This study was conducted to evaluate the protective effect of horse oil on ultraviolet B (UVB)-induced oxidative stress in human HaCaT keratinocytes. Horse oil significantly reduced UVB-induced intracellular reactive oxygen species and intracellular oxidative damage to lipids, proteins, and DNA. Horse oil absorbed light in the UVB range of the electromagnetic spectrum and suppressed the generation of cyclobutane pyrimidine dimers, a photoproduct of UVB irradiation. Western blotting showed that horse oil increased the UVB-induced Bcl-2/Bax ratio, inhibited mitochondria-mediated apoptosis and matrix metalloproteinase expression, and altered mitogen-activated protein kinase signaling-related proteins. These effects were conferred by increased phosphorylation of extracellular signal-regulated kinase 1/2 and decreased phosphorylation of p38 and c-Jun N-terminal kinase 1/2. Additionally, horse oil reduced UVB-induced binding of activator protein 1 to the matrix metalloproteinase-1 promoter site. These results indicate that horse oil protects human HaCaT keratinocytes from UVB-induced oxidative stress by absorbing UVB radiation and removing reactive oxygen species, thereby protecting cells from structural damage and preventing cell death and aging. In conclusion, horse oil is a potential skin protectant against skin damage involving oxidative stress.
Keywords: apoptosis; horse oil; oxidative stress; ultraviolet B radiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kim H., Youn K., Yun E.Y., Hwang J.S., Jeong W.S., Ho C.T., Jun M. Oleic acid ameliorates Aβ-induced inflammation by downregulation of COX-2 and iNOS via NFκB signaling pathway. J. Funct. Foods. 2015;14:1–11. doi: 10.1016/j.jff.2015.01.027. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

