Role of Protein Kinases in Hedgehog Pathway Control and Implications for Cancer Therapy
- PMID: 30934935
- PMCID: PMC6520855
- DOI: 10.3390/cancers11040449
Role of Protein Kinases in Hedgehog Pathway Control and Implications for Cancer Therapy
Abstract
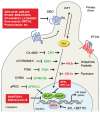
Hedgehog (HH) signaling is an evolutionarily conserved pathway that is crucial for growth and tissue patterning during embryonic development. It is mostly quiescent in the adult, where it regulates tissue homeostasis and stem cell behavior. Aberrant reactivation of HH signaling has been associated to several types of cancer, including those in the skin, brain, prostate, breast and hematological malignancies. Activation of the canonical HH signaling is triggered by binding of HH ligand to the twelve-transmembrane protein PATCHED. The binding releases the inhibition of the seven-transmembrane protein SMOOTHENED (SMO), leading to its phosphorylation and activation. Hence, SMO activates the transcriptional effectors of the HH signaling, that belong to the GLI family of transcription factors, acting through a not completely elucidated intracellular signaling cascade. Work from the last few years has shown that protein kinases phosphorylate several core components of the HH signaling, including SMO and the three GLI proteins, acting as powerful regulatory mechanisms to fine tune HH signaling activities. In this review, we will focus on the mechanistic influence of protein kinases on HH signaling transduction. We will also discuss the functional consequences of this regulation and the possible implications for cancer therapy.
Keywords: GLI; Hedgehog; Smoothened; cancer; phosphorylation; protein kinases; targeted therapy.
Conflict of interest statement
Unc-51 like kinase 3
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

