TRAILblazing Strategies for Cancer Treatment
- PMID: 30935038
- PMCID: PMC6521007
- DOI: 10.3390/cancers11040456
TRAILblazing Strategies for Cancer Treatment
Abstract
In the late 1990s, tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a member of the TNF-family, started receiving much attention for its potential in cancer therapy, due to its capacity to induce apoptosis selectively in tumour cells in vivo. TRAIL binds to its membrane-bound death receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5) inducing the formation of a death-inducing signalling complex (DISC) thereby activating the apoptotic cascade. The ability of TRAIL to also induce apoptosis independently of p53 makes TRAIL a promising anticancer agent, especially in p53-mutated tumour entities. Thus, several so-called TRAIL receptor agonists (TRAs) were developed. Unfortunately, clinical testing of these TRAs did not reveal any significant anticancer activity, presumably due to inherent or acquired TRAIL resistance of most primary tumour cells. Since the potential power of TRAIL-based therapies still lies in TRAIL's explicit cancer cell-selectivity, a desirable approach going forward for TRAIL-based cancer therapy is the identification of substances that sensitise tumour cells for TRAIL-induced apoptosis while sparing normal cells. Numerous of such TRAIL-sensitising strategies have been identified within the last decades. However, many of these approaches have not been verified in animal models, and therefore potential toxicity of these approaches has not been taken into consideration. Here, we critically summarise and discuss the status quo of TRAIL signalling in cancer cells and strategies to force tumour cells into undergoing apoptosis triggered by TRAIL as a cancer therapeutic approach. Moreover, we provide an overview and outlook on innovative and promising future TRAIL-based therapeutic strategies.
Keywords: TRAIL in cancer; TRAIL sensitising; TRAIL signalling; TRAIL-induced apoptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

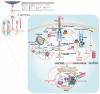
References
-
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.P., Nicholl J.K., Sutherland G.R., Smith T.D., Rauch C., Smith C.A., et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

