Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: a retrospective, multicentre cohort study
- PMID: 30935881
- PMCID: PMC6529612
- DOI: 10.1016/S2213-2600(18)30508-3
Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: a retrospective, multicentre cohort study
Abstract
Background: There is an urgent need for biomarkers to better stratify patients with idiopathic pulmonary fibrosis by risk for lung transplantation allocation who have the same clinical presentation. We aimed to investigate whether a specific immune cell type from patients with idiopathic pulmonary fibrosis could identify those at higher risk of poor outcomes. We then sought to validate our findings using cytometry and electronic health records.
Methods: We first did a discovery analysis with transcriptome data from the Gene Expression Omnibus at the National Center for Biotechnology Information for 120 peripheral blood mononuclear cell (PBMC) samples of patients with idiopathic pulmonary fibrosis. We estimated percentages of 13 immune cell types using statistical deconvolution, and investigated the association of these cell types with transplant-free survival. We validated these results using PBMC samples from patients with idiopathic pulmonary fibrosis in two independent cohorts (COMET and Yale). COMET profiled monocyte counts in 45 patients with idiopathic pulmonary fibrosis from March 12, 2010, to March 10, 2011, using flow cytometry; we tested if increased monocyte count was associated with the primary outcome of disease progression. In the Yale cohort, 15 patients with idiopathic pulmonary fibrosis (with five healthy controls) were classed as high risk or low risk from April 28, 2014, to Aug 20, 2015, using a 52-gene signature, and we assessed whether monocyte percentage (measured by cytometry by time of flight) was higher in high-risk patients. We then examined complete blood count values in the electronic health records (EHR) of 45 068 patients with idiopathic pulmonary fibrosis, systemic sclerosis, hypertrophic cardiomyopathy, or myelofibrosis from Stanford (Jan 01, 2008, to Dec 31, 2015), Northwestern (Feb 15, 2001 to July 31, 2017), Vanderbilt (Jan 01, 2008, to Dec 31, 2016), and Optum Clinformatics DataMart (Jan 01, 2004, to Dec 31, 2016) cohorts, and examined whether absolute monocyte counts of 0·95 K/μL or greater were associated with all-cause mortality in these patients.
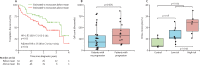
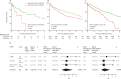
Findings: In the discovery analysis, estimated CD14+ classical monocyte percentages above the mean were associated with shorter transplant-free survival times (hazard ratio [HR] 1·82, 95% CI 1·05-3·14), whereas higher percentages of T cells and B cells were not (0·97, 0·59-1·66; and 0·78, 0·45-1·34 respectively). In two validation cohorts (COMET trial and the Yale cohort), patients with higher monocyte counts were at higher risk for poor outcomes (COMET Wilcoxon p=0·025; Yale Wilcoxon p=0·049). Monocyte counts of 0·95 K/μL or greater were associated with mortality after adjusting for forced vital capacity (HR 2·47, 95% CI 1·48-4·15; p=0·0063), and the gender, age, and physiology index (HR 2·06, 95% CI 1·22-3·47; p=0·0068) across the COMET, Stanford, and Northwestern datasets). Analysis of medical records of 7459 patients with idiopathic pulmonary fibrosis showed that patients with monocyte counts of 0·95 K/μL or greater were at increased risk of mortality with lung transplantation as a censoring event, after adjusting for age at diagnosis and sex (Stanford HR=2·30, 95% CI 0·94-5·63; Vanderbilt 1·52, 1·21-1·89; Optum 1·74, 1·33-2·27). Likewise, higher absolute monocyte count was associated with shortened survival in patients with hypertrophic cardiomyopathy across all three cohorts, and in patients with systemic sclerosis or myelofibrosis in two of the three cohorts.
Interpretation: Monocyte count could be incorporated into the clinical assessment of patients with idiopathic pulmonary fibrosis and other fibrotic disorders. Further investigation into the mechanistic role of monocytes in fibrosis might lead to insights that assist the development of new therapies.
Funding: Bill & Melinda Gates Foundation, US National Institute of Allergy and Infectious Diseases, and US National Library of Medicine.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Can monocytes predict prognosis of idiopathic pulmonary fibrosis?Lancet Respir Med. 2019 Jun;7(6):467-469. doi: 10.1016/S2213-2600(19)30050-5. Epub 2019 Mar 29. Lancet Respir Med. 2019. PMID: 30935880 No abstract available.
References
-
- Kim DS, Collard HR, King TE. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–292. - PMC - PubMed
- DS Kim, HR Collard, TE King. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc, 3, 2006, 285–292 - PMC - PubMed
-
- Raghu G, Collard HR, Egan JJ. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. - PMC - PubMed
- G Raghu, HR Collard, JJ Egan. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med, 183, 2011, 788–824 - PMC - PubMed
-
- Fujimoto H, Kobayashi T, Azuma A. Idiopathic pulmonary fibrosis: treatment and prognosis. Clin Med Insights Circ Respir Pulm Med. 2015;9(suppl 1):179–185. - PMC - PubMed
- H Fujimoto, T Kobayashi, A Azuma. Idiopathic pulmonary fibrosis: treatment and prognosis. Clin Med Insights Circ Respir Pulm Med, , 9 suppl 1 2015, 179–185 - PMC - PubMed
-
- Spagnolo P, Tonelli R, Cocconcelli E, Stefani A, Richeldi L. Idiopathic pulmonary fibrosis: diagnostic pitfalls and therapeutic challenges. Multidiscip Respir Med. 2012;7:42. - PMC - PubMed
- P Spagnolo, R Tonelli, E Cocconcelli, A Stefani, L Richeldi. Idiopathic pulmonary fibrosis: diagnostic pitfalls and therapeutic challenges. Multidiscip Respir Med, 7, 2012, 42 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- R35 HL144481/HL/NHLBI NIH HHS/United States
- U19 AI109662/AI/NIAID NIH HHS/United States
- P30 AR072579/AR/NIAMS NIH HHS/United States
- K12 HL120001/HL/NHLBI NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
- P01 HL092870/HL/NHLBI NIH HHS/United States
- R01 HL127805/HL/NHLBI NIH HHS/United States
- R01 AI125197/AI/NIAID NIH HHS/United States
- K08 HL130595/HL/NHLBI NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- R01 LM011369/LM/NLM NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

