The Amyloid Phenomenon and Its Significance in Biology and Medicine
- PMID: 30936117
- PMCID: PMC6996456
- DOI: 10.1101/cshperspect.a033878
The Amyloid Phenomenon and Its Significance in Biology and Medicine
Abstract
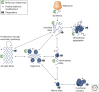
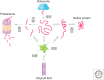
The misfolding of proteins is now recognized to be the origin of a large number of medical disorders. One particularly important group of such disorders is associated with the aggregation of misfolded proteins into amyloid structures, and includes conditions ranging from Alzheimer's and Parkinson's diseases to type II diabetes. Such conditions already affect over 500 million people in the world, a number that is rising rapidly, and at present these disorders cannot be effectively treated or prevented. This review provides an overview of this field of science and discusses recent progress in understanding the nature and properties of the amyloid state, the kinetics and mechanism governing its formation, the origins of its links with disease, and the manner in which its formation may be inhibited or suppressed. This latter topic is of particular importance, both to enhance our knowledge of the maintenance of protein homeostasis in living organisms and also to address the development of therapeutic strategies through which to combat the loss of homeostasis and the associated onset and progression of disease.
Copyright © 2020 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


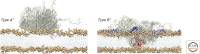

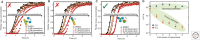

References
-
- Arosio P, Michaels TCT, Linse S, Månsson C, Emanuelsson C, Presto J, Johansson J, Vendruscolo M, Dobson CM, Knowles TPJ. 2016. Kinetic analysis reveals the diversity of microscopic mechanisms through which molecular chaperones suppress amyloid formation. Nat Commun 7: 10948 10.1038/ncomms10948 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
