Combining Monophosphoryl Lipid A (MPL), CpG Oligodeoxynucleotide (ODN), and QS-21 Adjuvants Induces Strong and Persistent Functional Antibodies and T Cell Responses against Cell-Traversal Protein for Ookinetes and Sporozoites (CelTOS) of Plasmodium falciparum in BALB/c Mice
- PMID: 30936155
- PMCID: PMC6529666
- DOI: 10.1128/IAI.00911-18
Combining Monophosphoryl Lipid A (MPL), CpG Oligodeoxynucleotide (ODN), and QS-21 Adjuvants Induces Strong and Persistent Functional Antibodies and T Cell Responses against Cell-Traversal Protein for Ookinetes and Sporozoites (CelTOS) of Plasmodium falciparum in BALB/c Mice
Abstract
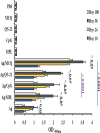
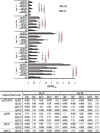
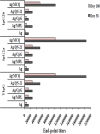
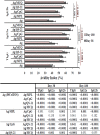
Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites (PfCelTOS) is an advanced vaccine candidate that has a crucial role in the traversal of the malaria parasite in both mosquito and mammalian hosts. As recombinant purified proteins are normally poor immunogens, they require to be admixed with an adjuvant(s); therefore, the objective of the present study was to evaluate the capacity of different vaccine adjuvants, monophosphoryl lipid A (MPL), CpG, and Quillaja saponaria Molina fraction 21 (QS-21), alone or in combination (MCQ [MPL/CpG/QS-21]), to enhance the immunogenicity of Escherichia coli-expressed PfCelTOS in BALB/c mice. This goal was achieved by the assessment of anti-PfCelTOS IgG antibodies (level, titer, IgG isotype profile, avidity, and persistence) and extracellular Th1 cytokines using an enzyme-linked immunosorbent assay (ELISA) on postimmunized BALB/c mouse sera and PfCelTOS-stimulated splenocytes, respectively. Also, an assessment of the transmission-reducing activity (TRA) of anti-PfCelTOS obtained from different vaccine groups was carried out in female Anopheles stephensi mosquitoes by using a standard membrane feeding assay (SMFA). In comparison to PfCelTOS alone, administration of PfCelTOS with three distinct potent Th1 adjuvants in vaccine mouse groups showed enhancement and improvement of PfCelTOS immunogenicity that generated more bias toward a Th1 response with significantly enhanced titers and avidity of the anti-PfCelTOS responses that could impair ookinete development in A. stephensi However, immunization of mice with PfCelTOS with MCQ mixture adjuvants resulted in the highest levels of induction of antibody titers, avidity, and inhibitory antibodies in oocyst development (88%/26.7% reductions in intensity/prevalence) in A. stephensi It could be suggested that adjuvant combinations with different mechanisms stimulate better functional antibody responses than adjuvants individually against challenging diseases such as malaria.
Keywords: CpG; MPL; PfCelTOS; Plasmodium falciparum; QS-21; adjuvants; malaria; vaccine.
Copyright © 2019 American Society for Microbiology.
Figures
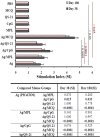


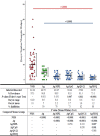
References
-
- World Health Organization. 2017. World malaria report 2017. World Health Organization, Geneva, Switzerland: https://apps.who.int/iris/bitstream/10665/259492/1/9789241565523-eng.pdf.
-
- World Health Organization. 2012. Global plan for insecticide resistance management in malaria vectors. World Health Organization, Geneva, Switzerland: https://www.who.int/malaria/publications/atoz/gpirm/en/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

