A Genomic Approach To Identify Klebsiella pneumoniae and Acinetobacter baumannii Strains with Enhanced Competitive Fitness in the Lungs during Multistrain Pneumonia
- PMID: 30936161
- PMCID: PMC6529660
- DOI: 10.1128/IAI.00871-18
A Genomic Approach To Identify Klebsiella pneumoniae and Acinetobacter baumannii Strains with Enhanced Competitive Fitness in the Lungs during Multistrain Pneumonia
Abstract
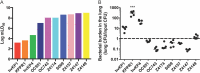
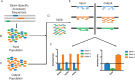
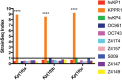
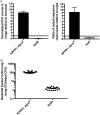
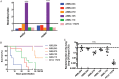
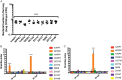
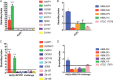
Microbial competition is most often studied at the genus or species level, but interstrain competition has been less thoroughly examined. Klebsiella pneumoniae is an important pathogen in the context of hospital-acquired pneumonia, and a better understanding of strain competition in the lungs could explain why some strains of this bacterium are more frequently isolated from pneumonia patients than others. We developed a barcode-free method called "StrainSeq" to simultaneously track the abundances of 10 K. pneumoniae strains in a murine pneumonia model. We demonstrate that one strain (KPPR1) repeatedly achieved a marked numerical dominance at 20 h postinoculation during pneumonia but did not exhibit a similar level of dominance in in vitro mixed-growth experiments. The emergence of a single dominant strain was also observed with a second respiratory pathogen, Acinetobacter baumannii, indicating that the phenomenon was not unique to K. pneumoniae When KPPR1 was removed from the inoculum, a second strain emerged to achieve high numbers in the lungs, and when KPPR1 was introduced into the lungs 1 h after the other nine strains, it no longer exhibited a dominant phenotype. Our findings indicate that certain strains of K. pneumoniae have the ability to outcompete others in the pulmonary environment and cause severe pneumonia and that a similar phenomenon occurs with A. baumannii In the context of the pulmonary microbiome, interstrain competitive fitness may be another factor that influences the success and spread of certain lineages of these hospital-acquired respiratory pathogens.
Keywords: Acinetobacter baumannii; Klebsiella pneumoniae; competition; pneumonia.
Copyright © 2019 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

