Defects in efferent duct multiciliogenesis underlie male infertility in GEMC1-, MCIDAS- or CCNO-deficient mice
- PMID: 30936178
- PMCID: PMC6503982
- DOI: 10.1242/dev.162628
Defects in efferent duct multiciliogenesis underlie male infertility in GEMC1-, MCIDAS- or CCNO-deficient mice
Abstract
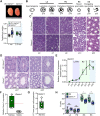
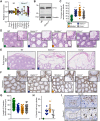
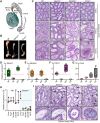
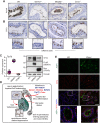
GEMC1 and MCIDAS are geminin family proteins that transcriptionally activate E2F4/5-target genes during multiciliogenesis, including Foxj1 and Ccno Male mice that lacked Gemc1, Mcidas or Ccno were found to be infertile, but the origin of this defect has remained unclear. Here, we show that all three genes are necessary for the generation of functional multiciliated cells in the efferent ducts that are required for spermatozoa to enter the epididymis. In mice that are mutant for Gemc1, Mcidas or Ccno, we observed a similar spectrum of phenotypes, including thinning of the seminiferous tubule epithelia, dilation of the rete testes, sperm agglutinations in the efferent ducts and lack of spermatozoa in the epididymis (azoospermia). These data suggest that defective efferent duct development is the dominant cause of male infertility in these mouse models, and this likely extends to individuals with the ciliopathy reduced generation of multiple motile cilia with mutations in MCIDAS and CCNO.
Keywords: CCNO; Efferent ducts; Fertility; GEMC1; MCIDAS; Multiciliated cells; P73; Testes; Transcription.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
-
- Amirav I., Wallmeier J., Loges N. T., Menchen T., Pennekamp P., Mussaffi H., Abitbul R., Avital A., Bentur L., Dougherty G. W. et al. (2016). Systematic analysis of CCNO variants in a defined population: implications for clinical phenotype and differential diagnosis. Hum. Mutat. 37, 396-405. 10.1002/humu.22957 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

