Oncogenic role of ABHD5 in endometrial cancer
- PMID: 30936746
- PMCID: PMC6421882
- DOI: 10.2147/CMAR.S188648
Oncogenic role of ABHD5 in endometrial cancer
Abstract
Background: Abhydrolase domain containing 5 (ABHD5) functions as a tumor suppressor in colorectal and prostate cancers. The aim of this study was to investigate the roles of ABHD5 in endometrial cancer.
Materials and methods: ABHD5 expression was detected in clinical samples by immunohistochemical staining. Cell proliferation and invasion were evaluated with the Cell Counting Kit-8 and Transwell assay, respectively. Western blotting was performed to analyze protein expression. Glucose uptake was assessed by 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose. Lactate production was detected by a lactate assay kit.
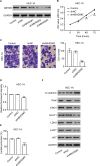
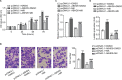
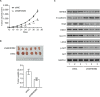
Results: In the present study, ABHD5 was overexpressed in endometrial cancer tissues, and its expression was closely correlated with the International Federation of Gynecology and Obstetrics (FIGO) stage and lymph node metastasis. In addition, we observed that the knockdown of ABHD5 inhibited cell proliferation, invasion, glucose uptake and lactate production in HEC-1A cells, which expressed high levels of ABHD5. Conversely, the opposite effects were observed when ABHD5 was ectopically expressed in Ishikawa cells, which had low levels of ABHD5. Furthermore, the changes in glycolysis regulators (enolase 1 [ENO1], glucose transporter 1 [GLUT1] and lactate dehydrogenase A [LDHA]) and epithelial-to-mesenchymal transition-related proteins (E-cadherin and Snail) in HEC-1A cells with ABHD5 knockdown were consistent with the effects of ABHD5 on glycolysis and cell invasion. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was increased, while the phosphorylated AKT (p-AKT) was decreased when ABHD5 was downregulated. Notably, treatment with the allosteric AKT inhibitor MK-2206 completely abolished the effects caused by ABHD5 overexpression in Ishikawa cells. Finally, ABHD5 knockdown potently suppressed tumor growth in vivo.
Conclusion: Overall, these results suggest that ABHD5 may play an oncogenic role in endometrial cancer via the AKT pathway.
Keywords: ABHD5; AKT; endometrial cancer; glycolysis.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Ali AT. Risk factors for endometrial cancer. Ceska Gynekol. 2013;78(5):448–459. - PubMed
-
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. - PubMed
-
- Bayley JP, Devilee P. The Warburg effect in 2012. Curr Opin Oncol. 2012;24(1):62–67. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

