Ombitasvir/paritaprevir/ritonavir plus ribavirin for 24 weeks in patients with HCV GT4 and compensated cirrhosis (AGATE-I Part II)
- PMID: 30937389
- PMCID: PMC6427060
- DOI: 10.1002/hsr2.92
Ombitasvir/paritaprevir/ritonavir plus ribavirin for 24 weeks in patients with HCV GT4 and compensated cirrhosis (AGATE-I Part II)
Abstract
Background and aims: AGATE-I Part I previously reported high sustained virologic response rates in hepatitis C genotype 4 patients with cirrhosis, with 12 and 16 weeks' treatment with a combination of two direct-acting antivirals, ombitasvir and paritaprevir (codosed with ritonavir), plus ribavirin. Part II, reported here, extended the trial to include a 24-week treatment arm to fully assess treatment duration in patients with chronic hepatitis C genotype 4 infection and compensated cirrhosis.
Methods: Enrollment took place between June and November of 2015. Treatment-naive and interferon-experienced patients with chronic hepatitis C genotype 4 infection and compensated cirrhosis were enrolled into Arm C; patients previously treated with a sofosbuvir-based regimen were enrolled into Arm D. All patients received a 24-week treatment with ombitasvir, paritaprevir, and ritonavir plus ribavirin. The primary outcome was the proportion of patients with a sustained virologic response (hepatitis C virus RNA < 25 IU/mL) at posttreatment week 12 in the intention-to-treat population. The safety population included all patients who received at least one dose of study drug.
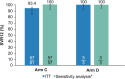
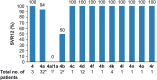
Results: In total, 64 patients were enrolled into AGATE-I Part II. Sustained virologic response at posttreatment week 12 was achieved in 57 of 61 patients (93.4%; 97.5% confidence interval, 92.6-97.7) in Arm C and 3 of 3 patients (100%) in Arm D. Two patients were missing SVR12 data, and two prematurely discontinued treatment. The most common adverse events for Arm C were fatigue (16 [26%]) and asthenia (15 [25%]). Results were comparable with those reported in Part I.
Conclusions: AGATE-I Part II indicates that extending treatment beyond 12 weeks in genotype 4-infected patients with compensated cirrhosis does not offer additional benefit.
Keywords: DAAs; HCV; compensated cirrhosis; genotype 4.
Conflict of interest statement
N.N.A., G.S., R.R., T.P.‐M., S.K.‐B., Y.Y., and N.M. are employees of AbbVie and may hold stock or stock options. T.A.: Clinical Investigator/Speaker/Consultant: AbbVie, Boehringer Ingelheim, BMS, Gilead Sciences, Janssen Pharmaceuticals, Merck Sharp & Dohme, Roche C.M.: Research grants: AbbVie, Gilead Sciences, Janssen; Consultant: AbbVie, Gilead Sciences, Janssen, Merck Sharp & Dohme, BMS S.P.: Consultant/Lecturer: BMS, Boehringer Ingelheim, Janssen, Gilead, MSD, Novartis, AbbVie; Grant/Research support: BMS, Gilead, Roche, MSD S.K.: Research grant: AbbVie M.G.: Advisor/Speaker: Janssen, MSD, BMS, Gilead, AbbVie; Grants: Gilead, AbbVie, MSD Y.H.: AbbVie, Gilead, Janssen, MSD, BMS I.E.: Advisor/Speaker: Gilead, AbbVie, MSD, BMS; Research grants: Gilead, AbbVie D.L.: Participation in clinical studies: AbbVie C.F.: Consultant: AbbVie, Gilead, Arrowhead, Humabs, MSD, Chiesi, Abivax; Research grants: Janssen, Cilag, Roche, BMS, Gilead, AbbVie M.R.: Participation in clinical studies: AbbVie A.O.: Consultant: AbbVie, MSD J.L.C.: Consultant and Lecturer: AbbVie, BMS, MSD, Gilead Sciences S.B.: Participation in clinical studies: AbbVie R.Q.: Participation in clinical studies: AbbVie
Figures
References
LinkOut - more resources
Full Text Sources
Medical

