AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking
- PMID: 30937469
- PMCID: PMC6502786
- DOI: 10.1007/s00018-019-03068-7
AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking
Abstract
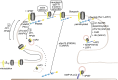
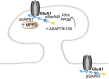
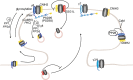
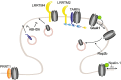
To correctly transfer information, neuronal networks need to continuously adjust their synaptic strength to extrinsic stimuli. This ability, termed synaptic plasticity, is at the heart of their function and is, thus, tightly regulated. In glutamatergic neurons, synaptic strength is controlled by the number and function of AMPA receptors at the postsynapse, which mediate most of the fast excitatory transmission in the central nervous system. Their trafficking to, at, and from the synapse, is, therefore, a key mechanism underlying synaptic plasticity. Intensive research over the last 20 years has revealed the increasing importance of interacting proteins, which accompany AMPA receptors throughout their lifetime and help to refine the temporal and spatial modulation of their trafficking and function. In this review, we discuss the current knowledge about the roles of key partners in regulating AMPA receptor trafficking and focus especially on the movement between the intracellular, extrasynaptic, and synaptic pools. We examine their involvement not only in basal synaptic function, but also in Hebbian and homeostatic plasticity. Included in our review are well-established AMPA receptor interactants such as GRIP1 and PICK1, the classical auxiliary subunits TARP and CNIH, and the newest additions to AMPA receptor native complexes.
Keywords: AMPA receptors; CNIH; GRIP1; MAGUK; PICK1; Synapse; TARP; Trafficking.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

