A Smartphone App to Assist Smoking Cessation Among Aboriginal Australians: Findings From a Pilot Randomized Controlled Trial
- PMID: 30938691
- PMCID: PMC6538311
- DOI: 10.2196/12745
A Smartphone App to Assist Smoking Cessation Among Aboriginal Australians: Findings From a Pilot Randomized Controlled Trial
Abstract
Background: Mobile health (mHealth) apps have the potential to increase smoking cessation, but little research has been conducted with Aboriginal communities in Australia.
Objective: We conducted a pilot study to assess the feasibility and acceptability and explore the effectiveness of a novel mHealth app to assist Aboriginal people to quit smoking.
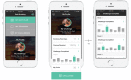
Methods: A pilot randomized controlled trial (RCT) and process evaluation comprising usage analytics data and in-depth interviews was conducted. Current Aboriginal smokers (>16 years old), who were willing to make a quit attempt in the next month, were recruited from Aboriginal Community Controlled Health Services and a government telephone coaching service. The intervention was a multifaceted Android or iOS app comprising a personalized profile and quit plan, text and in-app motivational messages, and a challenge feature allowing users to compete with others. The comparator was usual cessation support services. Outcome data collection and analysis were conducted blinded to treatment allocation. The primary outcome was self-reported continuous smoking abstinence verified by carbon monoxide breath testing at 6 months. Secondary outcomes included point prevalence of abstinence and use of smoking cessation therapies and services.
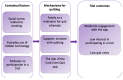
Results: A total of 49 participants were recruited. Competing service delivery priorities, the lack of resources for research, and lack of support for randomization to a control group were the major recruitment barriers. At baseline, 23/49 (47%) of participants had tried to quit in recent weeks. At 6-month follow-up, only 1 participant (intervention arm) was abstinent. The process evaluation highlighted low to moderate app usage (3-10 new users per month and 4-8 returning users per month), an average of 2.9 sessions per user per month and 6.3 min per session. Key themes from interviews with intervention participants (n=15) included the following: (1) the powerful influence of prevailing social norms around acceptability of smoking; (2) high usage of mobile devices for phone, text, and social media but very low use of other smartphone apps; (3) the role of family and social group support in supporting quit attempts; and (4) low awareness and utilization of smoking cessation support services. Despite the broad acceptability of the app, participants also recommended technical improvements to improve functionality, greater customization of text messages, integration with existing social media platforms, and gamification features.
Conclusions: Smoking cessation apps need to be integrated with commonly used functions of mobile phones and draw on social networks to support their use. Although they have the potential to increase utilization of cessation support services and treatments, more research is needed to identify optimal implementation models. Robust evaluation is critical to determine their impact; however, an RCT design may not be feasible in this setting.
Trial registration: Australian and New Zealand Clinical Trials Registry ACTRN12616001550493; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371792 (Archived by WebCite at http://www.webcitation.org/76TiV7HA6).
Keywords: mobile apps; oceanic ancestry group; smoking cessation.
©David Peiris, Lachlan Wright, Madeline News, Kris Rogers, Julie Redfern, Clara Chow, David Thomas. Originally published in JMIR Mhealth and Uhealth (http://mhealth.jmir.org), 02.04.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures





References
-
- Australian Bureau of Statistics. 2016. [2019-03-01]. 4714.0 - National Aboriginal and Torres Strait Islander Social Survey, 2014-15 https://www.abs.gov.au/ausstats/abs@.nsf/mf/4714.0 .
-
- Theo V, Bridget B, Lucy S, Alan D L. The Burden of Disease and Injury in Aboriginal and Torres Strait Islander Peoples. Brisbane: School of Population Health, The University of Queensland; 2007. p. 2003.
-
- Scott-Sheldon LA, Lantini R, Jennings EG, Thind H, Rosen RK, Salmoirago-Blotcher E, Bock BC. Text messaging-based interventions for smoking cessation: a systematic review and meta-analysis. JMIR Mhealth Uhealth. 2016;4(2):e49. doi: 10.2196/mhealth.5436. http://mhealth.jmir.org/2016/2/e49/ v4i2e49 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

