Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL
- PMID: 30938714
- PMCID: PMC6538317
- DOI: 10.1172/jci.insight.122627
Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL
Abstract
Background: Subgroups of patients with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) exhibit suboptimal outcomes after standard therapies, including oral kinase inhibitors. We and others have previously reported on safety and efficacy of autologous CD19-targeted CAR T-cells for these patients; here we report safety and long-term follow-up of CAR T-cell therapy with or without conditioning chemotherapy for patients with R/R CLL and indolent B-cell non-Hodgkin lymphoma (B-NHL).
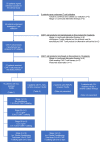
Methods: We conducted a phase 1 clinical trial investigating CD19-targeted CAR T-cells incorporating a CD28 costimulatory domain (19-28z). Seventeen of 20 patients received conditioning chemotherapy prior to CAR T-cell infusion. Five patients with CLL received ibrutinib at the time of autologous T-cell collection and/or CAR T-cell administration.
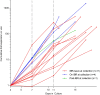
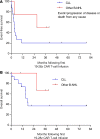
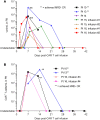
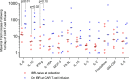
Results: This analysis included 16 patients with R/R CLL and 4 patients with R/R indolent B-NHL. Cytokine release syndrome (CRS) was observed in all 20 patients but grades 3 and 4 CRS and neurological events were uncommon (10% for each). Ex vivo expansion of T-cells and proportions of CD4+/CD8+ CAR T-cells with CD62L+CD127+ immunophenotype were significantly greater in patients on ibrutinib at leukapheresis. Three of 12 evaluable CLL patients receiving conditioning chemotherapy achieved CR (two had minimal residual disease-negative CR). All patients achieving CR remained progression-free at median follow-up of 53 months.
Conclusion: Conditioning chemotherapy and 19-28z CAR T-cells were acceptably tolerated across investigated dose levels in heavily pretreated patients with R/R CLL and indolent B-NHL, and a subgroup of patients achieved durable CR. Ibrutinib therapy may modulate autologous T-cell phenotype.
Trial registration: ClinicalTrials.gov NCT00466531.
Funding: Juno Therapeutics.
Keywords: Cancer immunotherapy; Clinical Trials; Immunology; Leukemias.
Conflict of interest statement
Figures
References
-
- Howlader N, et al, eds. SEER Cancer Statistics Review, 1975-2014. https://seer.cancer.gov/csr/1975_2014/ Updated April 2, 2019. Accessed April 2, 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

