Fructose malabsorption induces cholecystokinin expression in the ileum and cecum by changing microbiota composition and metabolism
- PMID: 30939042
- PMCID: PMC6988857
- DOI: 10.1096/fj.201801526RR
Fructose malabsorption induces cholecystokinin expression in the ileum and cecum by changing microbiota composition and metabolism
Abstract
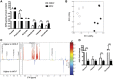
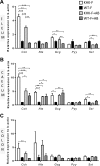
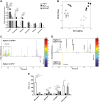
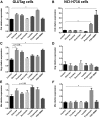
Current fructose consumption levels often overwhelm the intestinal capacity to absorb fructose. We investigated the impact of fructose malabsorption on intestinal endocrine function and addressed the role of the microbiota in this process. To answer this question, a mouse model of moderate fructose malabsorption [ketohexokinase mutant (KHK)-/-] and wild-type (WT) littermate mice were used and received a 20%-fructose (KHK-F and WT-F) or 20%-glucose diet. Cholecystokinin (Cck) mRNA and protein expression in the ileum and cecum, as well as preproglucagon (Gcg) and neurotensin (Nts) mRNA expression in the cecum, increased in KHK-F mice. In KHK-F mice, triple-label immunohistochemistry showed major up-regulation of CCK in enteroendocrine cells (EECs) that were glucagon-like peptide-1 (GLP-1)+/Peptide YY (PYY-) in the ileum and colon and GLP-1-/PYY- in the cecum. The cecal microbiota composition was drastically modified in the KHK-F in association with an increase in glucose, propionate, succinate, and lactate concentrations. Antibiotic treatment abolished fructose malabsorption-dependent induction of cecal Cck mRNA expression and, in mouse GLUTag and human NCI-H716 cells, Cck mRNA expression levels increased in response to propionate, both suggesting a microbiota-dependent process. Fructose reaching the lower intestine can modify the composition and metabolism of the microbiota, thereby stimulating the production of CCK from the EECs possibly in response to propionate.-Zhang, X., Grosfeld, A., Williams, E., Vasiliauskas, D., Barretto, S., Smith, L., Mariadassou, M., Philippe, C., Devime, F., Melchior, C., Gourcerol, G., Dourmap, N., Lapaque, N., Larraufie, P., Blottière, H. M., Herberden, C., Gerard, P., Rehfeld, J. F., Ferraris, R. P., Fritton, J. C., Ellero-Simatos, S., Douard, V. Fructose malabsorption induces cholecystokinin expression in the ileum and cecum by changing microbiota composition and metabolism.
Keywords: CCK; KHK; propionate.
Conflict of interest statement
The authors thank J. P. Furet (Micalis Institute, INRA, Jouy-en-Josas, France), F. Rouyer (Paris-Saclay Institute of Neuroscience, Université Paris Sud, CNRS, Université Paris–Saclay, Gif-sur-Yvette, France), and S. P. Shirazi-Beechey (Institute of Integrative Biology, University of Liverpool, United Kingdom) for sharing reagents and equipment. The authors are grateful to the INRA Migale bioinformatics platform (
Figures




References
-
- Marriott B. P., Cole N., Lee E. (2009) National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 139, 1228S–1235S - PubMed
-
- Tappy L., Lê K. A. (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 90, 23–46 - PubMed
-
- Jones H. F., Butler R. N., Brooks D. A. (2011) Intestinal fructose transport and malabsorption in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G202–G206 - PubMed
-
- Jones H. F., Burt E., Dowling K., Davidson G., Brooks D. A., Butler R. N. (2011) Effect of age on fructose malabsorption in children presenting with gastrointestinal symptoms. J. Pediatr. Gastroenterol. Nutr. 52, 581–584 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

