Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303
- PMID: 30939090
- PMCID: PMC6774813
- DOI: 10.1200/JCO.18.01994
Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303
Abstract
Purpose: Alliance/CALGB 50303 (NCT00118209), an intergroup, phase III study, compared dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) with standard rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) as frontline therapy for diffuse large B-cell lymphoma.
Patients and methods: Patients received six cycles of DA-EPOCH-R or R-CHOP. The primary objective was progression-free survival (PFS); secondary clinical objectives included response rate, overall survival (OS), and safety.
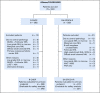
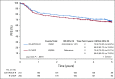
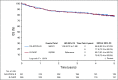
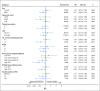
Results: Between 2005 and 2013, 524 patients were registered; 491 eligible patients were included in the final analysis. Most patients (74%) had stage III or IV disease; International Prognostic Index (IPI) risk groups included 26% IPI 0 to 1, 37% IPI 2, 25% IPI 3, and 12% IPI 4 to 5. At a median follow-up of 5 years, PFS was not statistically different between the arms (hazard ratio, 0.93; 95% CI, 0.68 to 1.27; P = .65), with a 2-year PFS rate of 78.9% (95% CI, 73.8% to 84.2%) for DA-EPOCH-R and 75.5% (95% CI, 70.2% to 81.1%) for R-CHOP. OS was not different (hazard ratio, 1.09; 95% CI, 0.75 to 1.59; P = .64), with a 2-year OS rate of 86.5% (95% CI, 82.3% to 91%) for DA-EPOCH-R and 85.7% (95% CI, 81.4% to 90.2%) for R-CHOP. Grade 3 and 4 adverse events were more common (P < .001) in the DA-EPOCH-R arm than the R-CHOP arm, including infection (16.9% v 10.7%, respectively), febrile neutropenia (35.0% v 17.7%, respectively), mucositis (8.4% v 2.1%, respectively), and neuropathy (18.6% v 3.3%, respectively). Five treatment-related deaths (2.1%) occurred in each arm.
Conclusion: In the 50303 study population, the more intensive, infusional DA-EPOCH-R was more toxic and did not improve PFS or OS compared with R-CHOP. The more favorable results with R-CHOP compared with historical controls suggest a potential patient selection bias and may preclude generalizability of results to specific risk subgroups.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Figures
Comment in
-
Reply to T.M. Weis et al.J Clin Oncol. 2019 Nov 1;37(31):2953. doi: 10.1200/JCO.19.01617. Epub 2019 Sep 18. J Clin Oncol. 2019. PMID: 31532721 No abstract available.
-
Dosing Vincristine in Dose-Adjusted EPOCH-R: To Cap or Not to Cap?J Clin Oncol. 2019 Nov 1;37(31):2952. doi: 10.1200/JCO.19.01259. Epub 2019 Sep 18. J Clin Oncol. 2019. PMID: 31532723 No abstract available.
References
-
- Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. - PubMed
-
- Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117–4126. - PubMed
-
- Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. - PubMed
-
- Pfreundschuh M, Trümper L, Österborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. - PubMed
-
- Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-Year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA233270/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180847/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- P50 CA097274/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- U10 CA049957/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
- UG1 CA233328/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

