Impact of a novel pharmacist-delivered behavioral intervention for patients with poorly-controlled diabetes: The ENhancing outcomes through Goal Assessment and Generating Engagement in Diabetes Mellitus (ENGAGE-DM) pragmatic randomized trial
- PMID: 30939143
- PMCID: PMC6445420
- DOI: 10.1371/journal.pone.0214754
Impact of a novel pharmacist-delivered behavioral intervention for patients with poorly-controlled diabetes: The ENhancing outcomes through Goal Assessment and Generating Engagement in Diabetes Mellitus (ENGAGE-DM) pragmatic randomized trial
Abstract
Background: Many factors contribute to suboptimal diabetes control including insufficiently-intensive treatment and non-adherence to medication and lifestyle. Determining which of these is most relevant for individual patients is challenging. Patient engagement techniques may help identify contributors to suboptimal adherence and address barriers (using motivational interviewing) and help facilitate choices among treatment augmentation options (using shared decision-making). These methods have not been used in combination to improve diabetes outcomes.
Objective: To evaluate the impact of a telephone-based patient-centered intervention on glycosylated hemoglobin (HbA1c) control for individuals with poorly-controlled diabetes.
Design: Two-arm pragmatic randomized control trial within an explanatory sequential mixed-methods design.
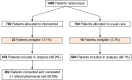
Subjects: 1,400 participants 18-64 years old with poorly-controlled type 2 diabetes.
Intervention: The intervention was delivered over the telephone by a clinical pharmacist and consisted of a 2-step process that integrated brief negotiated interviewing and shared decision-making to identify patient goals and options for enhancing diabetes management.
Main measures: The primary outcome was change in HbA1c. Secondary outcomes were medication adherence measures. Outcomes were evaluated using intention-to-treat principles; multiple imputation was used for missing values in the 12-month follow-up. We used information from pharmacist notes to elicit factors to potentially explain the intervention's effectiveness.
Key results: Participants had a mean age of 54.7 years (SD:8.3) and baseline HbA1c of 9.4 (SD:1.6). Change in HbA1c from baseline was -0.79 (SD:2.01) in the control arm and -0.75 (SD:1.76) in the intervention arm (difference:+0.04, 95%CI: -0.22, 0.30). There were no significant differences in adherence. In as-treated analyses, the intervention significantly improved diabetes control (-0.48, 95%CI: -0.91, -0.05). Qualitative findings provided several potential explanations for the findings, including insufficiently addressing patient barriers.
Conclusions: A novel telephone-based patient-centered intervention did not improve HbA1c among individuals with poorly-controlled diabetes, though as-treated analyses suggest that the intervention was effective for those who received it.
Trial registration: ClinicalTrials.gov NCT02910089.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Eric Wittbrodt is an employee of AstraZeneca. Julie C. Lauffenburger has received salary support for unrestricted research grants from Sanofi and Astra Zeneca. Niteesh K. Choudhry has received unrestricted research grants from Sanofi, Astra Zeneca, Merck, CVS Health, PhRMA Foundation, the Arnold Foundation and Medisafe. He is also a consultant to and holds equity in Ontiq, Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Misono AS, Cutrona SL, Choudhry NK, Fischer MA, Stedman MR, Liberman JN, et al. Healthcare information technology interventions to improve cardiovascular and diabetes medication adherence. The American journal of managed care. 2010;16(12 Suppl HIT):SP82–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical