Sodium thiocyanate treatment attenuates atherosclerotic plaque formation and improves endothelial regeneration in mice
- PMID: 30939159
- PMCID: PMC6445437
- DOI: 10.1371/journal.pone.0214476
Sodium thiocyanate treatment attenuates atherosclerotic plaque formation and improves endothelial regeneration in mice
Abstract
Introduction: Atherosclerotic plaque formation is an inflammatory process that involves the recruitment of neutrophil granulocytes and the generation of reactive oxygen species (ROS). ROS formation by myeloperoxidase, a key enzyme in H2O2 degradation, can be modulated by addition of sodium thiocyanate (NaSCN). However, the therapeutic use of NaSCN to counteract atherogenesis has been controversial, because MPO oxidizes NaSCN to hypothiocyanous acid, which is a reactive oxygen species itself. Therefore, this study aimed to investigate the effect of NaSCN treatment on atherogenesis in vivo.
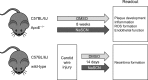
Methods: Apolipoprotein E knockout (ApoE-/-) mice on western-diet were treated with NaSCN for 8 weeks. Blood levels of total cholesterol, IL-10, and IL-6 were measured. Aortic roots from these mice were analyzed histologically to quantify plaque formation, monocyte, and neutrophil granulocyte infiltration. Oxidative damage was evaluated via an L-012 chemiluminescence assay and staining for chlorotyrosine in the aortic walls. Endothelial function was assessed by use of endothelium-dependent vasodilation in isolated aortic rings. Neointima formation was evaluated in wild-type mice following wire injury of the carotid artery.
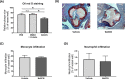
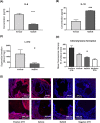
Results: NaSCN treatment of ApoE-/- mice lead to a reduction of atherosclerotic plaque size in the aortic roots but had no effect on monocyte or granulocyte infiltration. Serum levels of the pro-inflammatory cytokine IL-6 decreased whereas anti-inflammatory IL-10 increased upon NaSCN treatment. In our experiments, we found oxidative damage to be reduced and the endothelial function to be improved in the NaSCN-treated group. Additionally, NaSCN inhibited neointima formation.
Conclusion: NaSCN has beneficial effects on various stages of atherosclerotic plaque development in mice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- The World Health Organization. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016 Geneva, World Health Organization; 2018. Available from: https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1...
-
- Pasceri V, Cheng JS, Willerson JT, Yeh ET, Chang J. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001; 103: 2531–2534. - PubMed
-
- Ronald JA, Chen JW, Chen Y, Hamilton AM, Rodriguez E, Reynolds F, et al. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation. 2009; 120: 592–599. 10.1161/CIRCULATIONAHA.108.813998 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

