A Novel, Duodenal-Release Formulation of a Combination of Caraway Oil and L-Menthol for the Treatment of Functional Dyspepsia: A Randomized Controlled Trial
- PMID: 30939487
- PMCID: PMC6493684
- DOI: 10.14309/ctg.0000000000000021
A Novel, Duodenal-Release Formulation of a Combination of Caraway Oil and L-Menthol for the Treatment of Functional Dyspepsia: A Randomized Controlled Trial
Abstract
Objectives: We conducted a randomized, placebo-controlled trial, which evaluated a novel formulation of caraway oil and L-menthol using microsphere-based site-specific targeting (COLM-SST) vs placebo in patients with functional dyspepsia (FD).
Methods: Adult men and women with FD defined by Rome III criteria were recruited. Patients were randomized to COLM-SST (25 mg of caraway oil and 20.75 mg of L-menthol per capsule, at 2 capsules per dose, twice per day) or placebo. Efficacy was measured at 24 hours, 2 weeks, and 4 weeks. Patients were allowed to take concomitant medications for their FD throughout the trial, and rescue medicines were allowed, 48 hours after start of dosing.
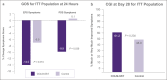
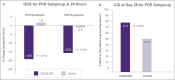
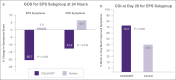
Results: Ninety-five patients were enrolled (mean age = 43.4 years; 75.8% women). At 24 hours, the active arm reported a statistically significant reduction in postprandial distress syndrome symptoms (P = 0.039), and a nonsignificant trend toward benefit of epigastric pain syndrome symptoms (P = 0.074). In patients with more severe symptoms, approximately 3 quarters of patients showed substantial global improvement (i.e., clinical global impressions), after 4 weeks of treatment, vs half in the control arm. These differences were statistically significant for patients with epigastric pain syndrome (P = 0.046), and trending toward significance for patients with postprandial distress syndrome (P = 0.091). There was no statistically significant difference between groups for Global Overall Symptom scores for the overall population at 2 and 4 weeks. Treatment emergent adverse events were mild to moderate, and no serious adverse events were reported.
Discussion: In patients taking their usual medications for FD, COLM-SST provided rapid relief (within 24 hours) and relief of severe FD symptoms. It was safe and well tolerated.
Figures
References
-
- Rome IV. Functional Gastrointestinal Disorders, Disorders of Gut-Brain Interaction, 4th edn, 2016.
-
- Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med 2015;373(19):1853–63. - PubMed
-
- Chen SL. A review of drug therapy for functional dyspepsia. J Dig Dis 2013;14(12):623–5. - PubMed
-
- Alhaider A, Al-Mofleh I, Mossa J, et al. Effect of Carum carvi on experimentally induced gastric mucosal damage in Wistar albino rats. Int J Pharmacol 2006;2(3):309–15.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

