Studying Heterotypic Cell⁻Cell Interactions in the Human Brain Using Pluripotent Stem Cell Models for Neurodegeneration
- PMID: 30939814
- PMCID: PMC6523455
- DOI: 10.3390/cells8040299
Studying Heterotypic Cell⁻Cell Interactions in the Human Brain Using Pluripotent Stem Cell Models for Neurodegeneration
Abstract
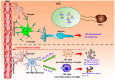
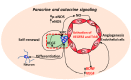
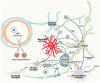
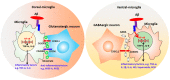
Human cerebral organoids derived from induced pluripotent stem cells (iPSCs) provide novel tools for recapitulating the cytoarchitecture of the human brain and for studying biological mechanisms of neurological disorders. However, the heterotypic interactions of neurovascular units, composed of neurons, pericytes (i.e., the tissue resident mesenchymal stromal cells), astrocytes, and brain microvascular endothelial cells, in brain-like tissues are less investigated. In addition, most cortical organoids lack a microglia component, the resident immune cells in the brain. Impairment of the blood-brain barrier caused by improper crosstalk between neural cells and vascular cells is associated with many neurodegenerative disorders. Mesenchymal stem cells (MSCs), with a phenotype overlapping with pericytes, have promotion effects on neurogenesis and angiogenesis, which are mainly attributed to secreted growth factors and extracellular matrices. As the innate macrophages of the central nervous system, microglia regulate neuronal activities and promote neuronal differentiation by secreting neurotrophic factors and pro-/anti-inflammatory molecules. Neuronal-microglia interactions mediated by chemokines signaling can be modulated in vitro for recapitulating microglial activities during neurodegenerative disease progression. In this review, we discussed the cellular interactions and the physiological roles of neural cells with other cell types including endothelial cells and microglia based on iPSC models. The therapeutic roles of MSCs in treating neural degeneration and pathological roles of microglia in neurodegenerative disease progression were also discussed.
Keywords: endothelial; heterotypic; mesenchymal stem cells; microglia; neural-vascular interactions; pluripotent stem cells.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Lee C.-T., Chen J., Kindberg A.A., Bendriem R.M., Spivak C.E., Williams M.P., Richie C.T., Handreck A., Mallon B.S., Lupica C.R. CYP3A5 mediates effects of cocaine on human neocorticogenesis: Studies using an in vitro 3D self-organized hPSC model with a single cortex-like unit. Neuropsychopharmacology. 2017;42:774. doi: 10.1038/npp.2016.156. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

