Safety and efficacy of the combination simeprevir-sofosbuvir in HCV genotype 1- and 4-mono-infected patients from the French ANRS CO22 hepather cohort
- PMID: 30940090
- PMCID: PMC6446259
- DOI: 10.1186/s12879-019-3923-5
Safety and efficacy of the combination simeprevir-sofosbuvir in HCV genotype 1- and 4-mono-infected patients from the French ANRS CO22 hepather cohort
Abstract
Background: Although real-life results of sofosbuvir/simeprevir have been extensively reported from the United States, data from other geographical areas are limited. In the French observational cohort, ANRS CO22 HEPATHER, 9432 patients were given the new oral antivirals from December 2013 to June 30, 2018. We report the results of sofosbuvir/simeprevir in genotypes 1- and 4-infected patients.
Methods: Demographics and history of liver disease were collected at entry in the cohort. Clinical, adverse events, and virological data were collected throughout treatment and post-treatment follow-up. The choice of treatment duration or addition of ribavirin was left up to the physician.
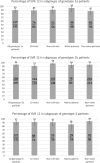
Results: Five hundred ninety-nine HCV (467 genotype 1 and 132 genotype 4) mono-infected, naïve for all oral-DAAs regimen patients were given sofosbuvir/simeprevir with (n = 63) or without ribavirin (n = 536) for 12 or 24 weeks; 56% had cirrhosis (4% decompensated) and 71% had prior treatment failure to interferon-based regimen. 7 patients (1.16%) were lost to follow-up. The overall SVR12 rate was 92.6%. The SVR12 was 90% in GT1a, 94.2% in GT1b and 91.6% in GT4 with no significant difference for genotype, treatment duration or ribavirin addition. Severity of liver disease was not associated with a lower SVR12 rate on multivariate analysis but was associated with a higher rate of severe side effects. Early treatment discontinuations were rare; no new safety signals were reported.
Conclusion: In this real life, observational, prospective cohort study, the 12-week sofosbuvir/simeprevir+/-ribavirin combination appears to be efficient and safe.
Trial registration: Trial registration with ClinicalTrials.gov NCT01953458 .
Keywords: Direct acting antivirals; Hepatitis C virus; Real life cohort; Simeprevir; Sofosbuvir.
Conflict of interest statement
Ethics approval and consent to participate
Written informed consent was obtained from each patient before enrolment. The protocol was performed in accordance with the Declaration of Helsinki and French law for biomedical research and was approved by the “CPP Ile de France 3” Ethics Committee (Paris, France) and the French Regulatory Authority (ANSM).
Consent for publication
not applicable.
Competing interests
The ANRS CO22 HEPATHER cohort is sponsored and funded by Inserm-ANRS and conducted in collaboration with Association Française pour l’étude du Foie (AFEF). The cohort received supports from ANR (Agence Nationale de la Recherche), DGS (Direction Générale de la Santé) and MSD, Janssen, Gilead, Abbvie, BMS, Roche. The public/private partnership is built in total transparency through a specific contract. The pharmaceutical companies are not involved in the scientific decisions.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Marcellin P, Pequignot F, Delarocque-Astagneau E, Zarski JP, Ganne N, Hillon P, et al. Mortality related to chronic hepatitis B and chronic hepatitis C in France: evidence for the role of HIV coinfection and alcohol consumption. J Hepatol. 2008;48(2):200–207. doi: 10.1016/j.jhep.2007.09.010. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical