Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis
- PMID: 30940209
- PMCID: PMC6444537
- DOI: 10.1186/s40425-019-0577-1
Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis
Abstract
Background: Current treatment guidelines for immune-mediated colitis (IMC) recommend 4 to 6 weeks of steroids as first-line therapy, followed by selective immunosuppressive therapy (SIT) (infliximab or vedolizumab) in patients who do not respond to steroids. We assessed the effect of early SIT introduction and number of SIT infusions on clinical outcomes.
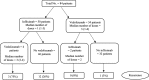
Methods: We performed a retrospective review of patients with IMC who received SIT at The University of Texas MD Anderson Cancer Center between January and December 2018. Logistic regression analyses were used to assess associations between clinical outcomes and features of IMC.
Results: Of the 1459 patients who received immune checkpoint inhibitors, 179 developed IMC of any grade; 84 of these 179 patients received SIT. Of the 84 patients who received SIT, 79% were males, and the mean age was 60 years (standard deviation, 14). Compared with patients who received SIT > 10 days after IMC onset, patients who received early SIT (≤10 days) required fewer hospitalizations (P = 0.03), experienced steroid taper failure less frequently (P = 0.03), had fewer steroid tapering attempts (P < 0.01), had a shorter course of steroid treatment (P = 0.09), and had a shorter duration of symptoms (P < 0.01). Patients who received one or two infusions of SIT achieved histologic remission less frequently (P = 0.09) and had higher fecal calprotectin levels after SIT (P = 0.01) compared with patients who received three or more infusions. Risk factors for IMC recurrence after weaning off steroids included: 1) needing multiple hospitalizations, 2) experiencing steroid taper failure after SIT, 3) receiving infliximab rather than vedolizumab, 4) receiving fewer than three infusions of SIT, 5) having higher fecal calprotectin levels after SIT, and 6) receiving a longer course of steroids, hospitalization and IMC symptoms. Unsuccessful weaning from steroids after SIT was associated with high IMC grades; multiple hospitalizations; steroid-resistant IMC; long interval from IMC to SIT initiation; and long duration of steroids, IMC symptoms, and hospitalization.
Conclusion: SIT should be introduced early in the disease course of IMC instead of waiting until failure of steroid therapy or steroid taper. Patients who received three or more infusions of SIT had more favorable clinical outcomes.
Keywords: Colitis; Diarrhea; Immune checkpoint inhibitors; Immunotherapy; Infliximab; Vedolizumab.
Conflict of interest statement
Ethics approval and consent to participate
The ethics approval of this study was granted by the IRB committee at the University of Texas MD Anderson Cancer Center (PA18–0472). The consent was waived for this study.
Consent for publication
This study was granted waiver for consent.
Competing interests
The authors declare that they have no conflict of interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. - DOI - PMC - PubMed
-
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (sitc) toxicity management working group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials