Checkpoint Inhibitors
- PMID: 30940340
- PMCID: PMC6454802
- DOI: 10.3238/arztebl.2019.0119
Checkpoint Inhibitors
Abstract
Background: Treatment with checkpoint inhibitors such as anti-programmed death-1 (anti-PD-1), anti-PD-ligand 1 (anti-PD-L1), and anti-cytotoxic T-lymphocyte antigen-4 (anti-CTLA-4) antibodies can prolong the survival of cancer patients, but it also induces autoimmune side effects in 86-96% of patients by activating the immune system. In 17-59% of patients, these are severe or even life-threatening.
Methods: This review is based on pertinent articles retrieved by a search in PubMed and on an evaluation of a side-effect registry.
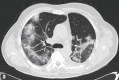
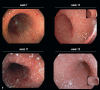
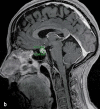
Results: Checkpoint-inhibitor-induced autoimmune side effects manifest themselves in all organ systems, most commonly as skin lesions (46-62%), autoimmune colitis (22-48%), autoimmune hepatitis (7-33%), and endocrinopathies (thyroiditis, hypophysitis, adrenalitis, diabetes mellitus; 12-34%). Rarer side effects include pneumonitis (3-8%), nephritis (1-7%), cardiac side effects including cardiomyositis (5%), and neurological side effects (1-5%). Severe (sometimes lethal) side effects arise in 17-21%, 20-28%, and 59% of patients undergoing anti-PD-1 and anti- CTLA-4 antibody treatment and the approved combination therapy, respectively. With proper monitoring, however, these side effects can be recognized early and, usually, treated with success. Endocrine side effects generally require long-term hormone substitution. Patients who have stopped taking checkpoint inhibitors because of side effects do not show a poorer response of their melanoma or shorter survival in comparison to patients who continue to take checkpoint inhibitors.
Conclusion: The complex management of checkpoint-inhibitor-induced side effects should be coordinated in experienced centers. The creation of an interdisciplinary "tox team" with designated experts for organ-specific side effects has proven useful. Prospective registry studies based on structured documentation of side effects in routine clinical practice are currently lacking and urgently needed.
Figures

Comment in
-
Quality, Rather Than Quantity, of Life Is the Crucial Criterion.Dtsch Arztebl Int. 2019 May 17;116(20):363. doi: 10.3238/arztebl.2019.0363a. Dtsch Arztebl Int. 2019. PMID: 31288920 Free PMC article. No abstract available.
References
-
- Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. - PubMed
-
- Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18:1202–1210. - PubMed
-
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

