Positive allosteric modulation of the type 1 cannabinoid receptor reduces the signs and symptoms of Huntington's disease in the R6/2 mouse model
- PMID: 30940536
- PMCID: PMC6544167
- DOI: 10.1016/j.neuropharm.2019.03.033
Positive allosteric modulation of the type 1 cannabinoid receptor reduces the signs and symptoms of Huntington's disease in the R6/2 mouse model
Abstract
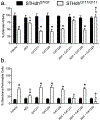
Huntington's disease (HD) is an inherited progressive neurodegenerative disease characterized by motor, cognitive, and behavioural changes. One of the earliest changes to occur in HD is a reduction in cannabinoid 1 receptor (CB1) levels in the striatum, which is strongly correlated with HD pathogenesis. CB1 positive allosteric modulators (PAM) enhance receptor affinity for, and efficacy of activation by, orthosteric ligands, including the endocannabinoids anandamide and 2-arachidonoylglycerol. The goal of this study was to determine whether the recently characterized CB1 allosteric modulators GAT211 (racemic), GAT228 (R-enantiomer), and GAT229 (S-enantiomer), affected the signs and symptoms of HD. GAT211, GAT228, and GAT229 were evaluated in normal and HD cell models, and in a transgenic mouse model of HD (7-week-old male R6/2 mice, 10 mg/kg/d, 21 d, i.p.). GAT229 was a CB1 PAM that improved cell viability in HD cells and improved motor coordination, delayed symptom onset, and normalized gene expression in R6/2 HD mice. GAT228 was an allosteric agonist that did not enhance endocannabinoid signaling or change symptom progression in R6/2 mice. GAT211 displayed intermediate effects between its enantiomers. The compounds used here are not drugs, but probe compounds used to determine the potential utility of CB1 PAMs in HD. Changes in gene expression, and not protein, were quantified in R6/2 HD mice because HD pathogenesis is associated with dysregulation of mRNA levels. The data presented here provide the first proof of principle for the use of CB1 PAMs to treat the signs and symptoms of HD.
Keywords: Allosteric modulator; Cannabinoid; G protein-coupled receptor; Huntington's disease; Neurodegeneration; Type 1 cannabinoid receptor.
Copyright © 2019. Published by Elsevier Ltd.
Conflict of interest statement
Conflict of Interest Statement
None declared.
Figures



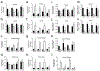
References
-
- Shannon KM, Fraint A (2015): Therapeutic advances in Huntington’s disease. Mov Disord 30: 1539–1546. - PubMed
-
- Denovan-Wright EM, Robertson HA (2000): Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington’s disease mice. Neuroscience 98: 705–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

