Targeting Glutamine Metabolism and Redox State for Leukemia Therapy
- PMID: 30940653
- PMCID: PMC6642698
- DOI: 10.1158/1078-0432.CCR-18-3223
Targeting Glutamine Metabolism and Redox State for Leukemia Therapy
Abstract
Purpose: Acute myeloid leukemia (AML) is a hematologic malignancy characterized by the accumulation of immature myeloid precursor cells. AML is poorly responsive to conventional chemotherapy and a diagnosis of AML is usually fatal. More effective and less toxic forms of therapy are desperately needed. AML cells are known to be highly dependent on the amino acid glutamine for their survival. These studies were directed at determining the effects of glutaminase inhibition on metabolism in AML and identifying general weaknesses that can be exploited therapeutically.
Experimental design: AML cancer cell lines, primary AML cells, and mouse models of AML and acute lymphoblastic leukemia (ALL) were utilized.
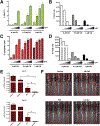
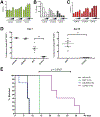
Results: We show that blocking glutamine metabolism through the use of a glutaminase inhibitor (CB-839) significantly impairs antioxidant glutathione production in multiple types of AML, resulting in accretion of mitochondrial reactive oxygen species (mitoROS) and apoptotic cell death. Moreover, glutaminase inhibition makes AML cells susceptible to adjuvant drugs that further perturb mitochondrial redox state, such as arsenic trioxide (ATO) and homoharringtonine (HHT). Indeed, the combination of ATO or HHT with CB-839 exacerbates mitoROS and apoptosis, and leads to more complete cell death in AML cell lines, primary AML patient samples, and in vivo using mouse models of AML. In addition, these redox-targeted combination therapies are effective in eradicating ALL cells in vitro and in vivo.
Conclusions: Targeting glutamine metabolism in combination with drugs that perturb mitochondrial redox state represents an effective and potentially widely applicable therapeutic strategy for treating multiple types of leukemia.
©2019 American Association for Cancer Research.
Conflict of interest statement
Conflicts of Interest
The authors declare no conflicts of interest.
Figures



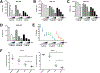
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

